Beer, Wine and
the Colloids Which
Make it All Possible
David Bohnsack
Dannis Prasadi
Jeffrey Reinig
Lisa Wetzel
We can find alcohols throughout our daily lives, and they are used widely in industry and home, including as a component of beer and wine. Of the many compounds that are alcohols, only one, ethyl alcohol (or ethanol) is the component of the beer and wine that we drink.
Beer typically has ethanol content of 4 –5 weight %, while wine has 10 - 14 weight % depends on its type. As beer and wine composed mainly of water-ethanol mixtures are varied with other flavored components, they enhance many surface and colloidal phenomena involving all of those components.
The flavor profile of beer and wine varies widely. Even there are hundreds of flavor and aroma descriptors for them, and the balance of these characteristics along with appearance and body is what gives beer and wine its complexity, but the basic flavor requirements are the same: bitter, sweet, salty, with unique aroma. Each ingredient in beer and wine contributes to the different flavor and aroma to the final product.
Another important process
in beer and wine production that can lead to the surface and colloidal
phenomena is carbonation. Carbonation is obtained naturally during the
fermentation of sugar into alcohol or artificially by injecting carbon dioxide
into the liquid. Carbon dioxide not only contributes to perceived
“fullness" or "body" and enhances foaming potential, but it also
acts as a flavor enhancer and plays an important role in extending the shelf
life of the product. Carbonation occurs when carbon dioxide gas is added to a
water-based liquid. The carbon dioxide reacts chemically with the water
molecules to form carbonic acid as follows:
CO2 + H2O => H2CO3
(carbonic acid)
This reaction works well
when the water is under pressure, but when the pressure is released, the
reaction tends to reverse. The level of dissolved carbon dioxide in beer
following primary fermentation varies as a result of a number of parameters such
as temperature, pressure, yeast, type of fermentation vessel, and initial wort
clarity. Typically, carbon dioxide levels range from 1.2 to 1.7 volumes
of carbon dioxide per volume of beer for non-pressurized fermentations.
Therefore, carbon dioxide levels need adjustment, unless the beer has undergone
a traditional lagering. Common practice is to raise the carbon dioxide
level between 2.2 and 2.8 volumes of carbon dioxide per volume of beer prior to
packaging (Goldammer).
The definition of wine is a beverage resulting from the fermentation by yeasts of the juice of the grape, with appropriate (and legal) processing and additions. Wine classifications are based, in order, on content of alcohol, dissolved gas, sugar, and then on color, and eventually on grape variety, geographical origin, and age (Ullmann, 270-275):
(1) Table Wines (with ethanol £ 14 volume %)
(a) Still Table wines (contain less than 3.92g CO2 per liter)
o Dry, Still Table wines. Dry is an enological term for wines having either no perceptible sweetness and/or wines containing no fermentable sugar, the residual concentrations of glucose and fructose should be zero or nearly so.
§ Dry, Still, Varietal table wines: based on grape variety, and indeed may supersede the classification based on color.
o Nondry, Still table wines: experts assign 5g/L glucose in wine as the sensory recognition threshold for sweetness. For the customer, the more operative definition of dryness in wine is the lack of perceptible sweetness.
(b) Sparkling Table wines: contain appreciable concentrations of carbon dioxide.
o Dry Sparkling Table Wines
o Sweet Sparkling Wines
(2) Dessert/ Appetizer Wines.
A very conspicuous difference between table and dessert/appetizer wines, is that the latter microbiologically stable in the presence of air and fermentable sugar (glucose and fructose). This is due to the higher content of ethanol.
(a) Sweet Dessert/Appetizer Wines
(b) Maderized wines: the term maderized denotes those wines, which have been intentionally oxidized by various methods to produce a characteristic flavor.
a. Oxidized by Film Yeast
b. Oxidized with Age
c. Oxidized by Heat Treatment
(c) Flavored Dessert/Appetizer Wines
a. Vermouths
b. Specialty and Proprietary Dessert/Appetizer Wines: An important class, those to which particular herbs or flavors are added to give the product a distinctive, and often a proprietary, character.
(3) Dealcoholized Wines
(a) Nonalcoholic wines: lowered content of ethanol.
(b) Wines with Partial Removal of Ethanol
There are really only two main types of beer: Ales and Lager. The branch of the beer families corresponds to the type of yeast used. There are ale yeast and lager yeast, and these yeasts in turn determine what other ingredients and techniques will work in a given recipe (Nachel, 11).
Ales tend to have heavier bodies, more alcohol, a darker hue, and are cloudier than lager. Ales are the older, distinguished, traditional brews of the world, predating lagers by thousands of years, whereas lagers are a relatively modern invention, dating from the mid-19th century.
The name lager is taken from the German word meaning "to store". Most of the mass-produced of the world are lagers. Lager has a cleaner taste and appearance. Lagers also are less hoppy, maltier and have a lighter body than ales.
Like wine, there is also beer with lowered ethanol content. The maximum content of ethanol for this type of beer is 0.5%, which will naturally change the complexity and balance of flavors of the beer.
Ingredients
The ingredients of beer are malted barley, hops, yeast and water. Barley is the seed of a grain that looks a lot like wheat. Before barley can be used to make beer, it must be malted, which involves a natural conversion process. There are two basic types of malting barley. One produces two rows of kernels on each stalk, and the other yields six rows on each stalk. The flavor of the two varieties differs. Two-row barley malt produces a smoother, sweeter-tasting beer, and six-row barley produces a crisper, snappier flavor. First, the barley must be allowed to germinate, or start to sprout. This is done by soaking the barley in water for several days, and then draining the barley and holding it at about 60 °F (15.5 °C) for five days. This allows the husk to open and barley to start to sprout; at this point it is called green malt. Like all seeds, the barley contains nutrients that can sustain the growing seed until it can produce its own nutrients using photosynthesis. During the germination process, enzymes released by the plant convert these nutrients (which are starches) into sugars that can feed the plant while it grows. The key to the malting process is to stop the germination of the barley at a point when the sugar-producing enzymes are present but most of the starch is still unconverted. Eventually, these enzymes will produce the sugars that will feed the yeast to make alcohol in the beer. After this natural process has released the enzymes, the green malt is dried by gradually raising the temperature. The intensity of the malt flavor and color depends on how high the temperature is raised during the drying process. One final step must be completed -- removing any small roots that formed during germination -- and the malted barley is ready to begin the brewing process. Most breweries buy barley that has already been malted to their specifications (Nice).
The hops used to make beer are the flower of the hop vine, which is a member of the hemp family (Cannabaceae). Hops contain acids, which give beer its bitterness, as well as oils that give beer some of its flavor and aroma. Adding hops to beer also inhibits the formation of certain bacteria that can spoil the beer. There are many different kinds of hops, each of which gives a different taste, aroma and amount of bitterness to the beer it is used in.
Yeast is the single-celled microorganism that is responsible for creating the alcohol and carbon dioxide found in beer. There are many different kinds of yeasts used to make beer; and just as the yeast in a sourdough starter gives sourdough bread its distinctive flavor, different types of beer yeast help to give beer its various tastes, mainly to its crisp, clean taste and aroma. There are two main categories of beer yeast: ale yeast and lager yeast. Ale yeast is top fermenting, meaning it rises near the surface of the beer during fermentation, and typically prefers to ferment at temperatures around 70 °F (21 °C). Lager yeasts are bottom fermenting. They ferment more slowly and prefer colder temperatures, around 50 °F (10 °C) (Nice).
The brewing water has long been recognized as making an important contribution to the flavor of beer. This is especially important since water composes more than 90% of the beer. In the past, the mineral content of water influenced greatly the flavor of the final beer and was specific to the region of the earth from which it came. Today, almost any water can be chemically adjusted to create the exact style of beer desired, although pure water supplies are still prized greatly. Some beer styles are also spiced with ingredients such as coriander, curacaos, and others. As the result, the composition of the beer typically 92 – 93% of water, 4 – 5.5 % alcohol, and 2 –3 % other flavored components.
Main ingredient for winemaking is grape. The grapevines from which wines are made are all members of the genus Vitis and belong to the botanical family Vitaceae. The “wine grape” (Vitis vinifera) does not tolerate extreme climatic conditions. The varieties are simply divided into “white” and “red” grapes. There are some 15,000 named sub-species of V. vinifera, but probably only 1,000 have been exploited for wine production, and of these, perhaps only a few dozen are actually in use commercially (Ullmann, 292).
The most important attribute of V. vinifera – besides the properties of its juices which allow the production of a beverage of high ethanol content and high acidity, giving wine its microbiological stability – is that the product does not have overstated flavors, but rather a sensitivity that allows the flavors to remain bland. The tannin and anthocyanin pigments, which provide the red color and astringent and bitter flavors to wines are highly localized in the skin of V. vinifera. For red wine production these elements must be extracted, which is generally done by fermenting these grapes “on the skins”, the heat and ethanol releasing them from the skin cells. For white wine production from white grapes, the skins are generally removed before fermentation, to decrease the bitter and astringent components.
The main components of wine are water and ethanol. High concentrations of ethanol in wine may contribute a slight sweet taste and also give a slight burning sensation, described as hot. Wine shows, of course, all of the physical characteristics (densities, viscosities, surface tensions, and boiling points) associated with water-ethanol mixtures. The determination of the density of wine is about the only physical measurement routinely made, but this measurement is of paramount importance for the winemaker. Except for temperature, other physical measurements are almost never used or even made.
Density measurements are performed not only on the wine and wine extract, but are also taken before, during, and after fermentation, to determine the fermentation behavior. The density of ethanol, under standard conditions, is 0.79 g/mL. Thus, the densities of table wines are usually lower than that of water, depending on the concentration of the concentration of ethanol in the wine. The soluble solids in the extract will tend to increase the density. A very dry table wine with 14 volume % ethanol and 2% extract might have a density as low as 0.994; while a Port Wine with 18 volume % ethanol and 7 or 8% of residual sugar might have a density of 1.024 (Ullmann, 278). Density measurements are important in assessments of ripeness of grapes and of the course of grape juice fermentation.
In addition to the density, only the temperature of wine is measured habitually by winemakers. The temperature is needed first of all to correct the density values. The temperature reading is also important for controlling both the alcoholic and the malolactic fermentations, but also to assure correct storage of the wine in the cellar and in bottles.
The other components are sugars, acid, other alcohols, and phenolics, all being present in the magnitude of grams per liter. But these components are important in several aspects of the sensory qualities of the wines; in its sweetness or acidity, in its astringency or bitterness, in its mouth feel (the viscosity or body), certainly in its color, and its aging potential. A rough comparison of typical compositions of wines without unusual additions is given in the following table (Kirk-Othmer, 745):
Wt %
|
Components |
Table Wines |
Dessert Wines |
||
|
White |
Red |
White |
Red |
|
|
Water |
87 |
87 |
76 |
74 |
|
Ethanol |
10 |
10 |
14 |
14 |
|
Other volatiles |
0.04 |
0.04 |
0.05 |
0.05 |
|
Extract |
2.6 |
2.7 |
10.1 |
12.2 |
|
Sugar |
0.05 |
0.05 |
8 |
10 |
|
Pectin and related substances |
0.3 |
0.3 |
0.25 |
0.25 |
|
Glycerol and related substances |
1.1 |
1.1 |
0.9 |
0.9 |
|
Acids |
0.7 |
0.6 |
0.5 |
0.5 |
|
Ash |
0.2 |
0.2 |
0.2 |
0.2 |
|
Phenols |
0.01 |
0.2 |
0.01 |
0.2 |
|
Amino acids and related substances |
0.25 |
0.25 |
0.2 |
0.2 |
|
Fats, terpenoid |
0.01 |
0.02 |
0.01 |
0.02 |
|
Miscellaneous and vitamins |
0.01 |
0.01 |
0.01 |
0.01 |
|
Total |
100 |
100 |
100 |
100 |
Table 1: Typical
Composition of Wine
Extract is composed mostly of glycerol and organic acids. Residual fermentable sugars, as dissolve solids, may or may not be used included in the extract depending on the purposes of the measurements.
Several types of colloids used in wine can
be identified. They are:
a) Sugars.
o Glucose: The other name is dextrose. It is blood sugar; corn sugar; grape sugar; starch sugar. Naturally, it occurs in plant tissues and is obtained from the complete hydrolysis of starch. The degree of sweetness of glucose is 70% of that of table sugar (sucrose).
o Fructose: It is a fruit sugar and it is also called levulose. It is a monosaccharide sugar found naturally in fruits and honey. The source is from the hydrolysis of sucrose (table sugar). It is very sweet and the degree of sweetness is 170% in relation to table sugar (100%).
(b) Amino Acids
A group of organic compounds, known as the building blocks of proteins.
(c) Pectin
Pectin is a structural
polysaccharide. Grape juice contains high concentrations of pectins, which
consists mostly of polymeric chains of galacturonic acid and its methyl esters.
Most of the pectins are precipitated during fermentation by the increasing
concentrations of ethanol. Finished wines are often devoid of pectin, having
been treated before or after the fermentation with pectinase enzymes.
Production Process
Brewing begins with malted barley that is milled and mixed with hot water to form a mash. During mashing, the malt starches are converted to sugars. The sugar rich water is then strained through the bottom of the mash and is now called wort. The wort then goes to the brew kettle where it is brought to a boil. During this stage, hops are added at different times during the boil for either bitterness or aroma. The wort is then cooled and aerated, and brewers' yeast is added for fermentation. The yeast produces alcohol and carbon dioxide and other byproducts from the sweet wort. After fermentation, the "green beer" undergoes maturation. The last step in the brewing process is filtration, and then carbonation. Next, the beer is moved to a holding tank where it stays until it is bottled or kegged (Goldammer).
Winemaking can be divided into four basic phases. The first phase consists of finding a source of high quality fruit and making sure the grapes are harvested in an optimum condition. Buying small quantities of high quality fruit is not easy, and this is the most difficult winemaking phase for home winemakers. The second phase consists of fermenting the grapes into wine. Winemakers manage the fermentation by controlling several different fermentation parameters such as temperature, skin contact time, pressing technique, etc. During the third phase, the new wine is clarified and stabilized. Winemakers clarify wine by fining, racking, and filtration. Wine is stabilized by removing excessive protein and potassium hydrogen tartrate (potassium bi-tartrate). These materials must be removed to prevent them from precipitating out of the wine later. In the fourth phase of winemaking, the winemaker ages the wine. Most high quality wines are aged in bulk and then for an additional time in the bottle. Winemakers have an active role throughout the lengthy bulk aging process. Wines are smelled, tasted, and measured every few weeks, and any needed adjustments are made promptly. Except for the first phase, the other three winemaking phases overlap each other. New wine starts to clarify toward the end of the fermentation period. Some tartrates precipitate out during primary fermentation, and the wine becomes more stable. Of course, wine is aging throughout the winemaking process. Each phase makes a specific contribution to wine characteristics, but the first phase has the greatest influence on wine quality.
There are some special issues in the process of brewing and winemaking that deal with our interests in colloidal and surface phenomena. For example, the removal of ethanol in the nonalcoholic beer and wine brings a problem in microbiological stability. The issues discussed are filtration, bottling, and flavor.
Filtration
Making quality beer and wine involves choosing the right raw materials to running each of the unit operations in a manner that does not compromise the quality of the product. Molecular separations are needed to concentrate and purify a wide variety of biological and chemical process fluids, and to remove very fine particulate contamination for fluid clarification, including beer and wine clarification.
Bottling
The most important thing about the bottling and kegging process is to keep the beer from being contaminated by stray yeasts, and to keep oxygen away from the beer. These are the main things that can reduce the shelf life of beer. The greater the amount of oxygen the less stable the beer, therefore care must be taken not to introduce oxygen during bottling. In the USA the headspace in bottles fell from 9.2 ml per 12 oz (355 ml) bottle in 1935 to 0.4 ml per 12 oz (355 ml) bottle in 1956. This greatly improved the colloidal stability of the beer. This difference is solely attributable to oxygen. The optimum head- space was less than 1% of the volume of the beer. The temperature of the beer when bottled did not affect the colloidal stability but storage temperature after bottling was important. In general, higher storage temperatures led to poorer stabilities. Beer in larger bottles has less exposure to air in the headspace relative to the total volume of beer in the bottle, so the larger bottle reduces the risk of over oxidation.
Flavor
Bad taste of the beer and wine product can be caused by volatile, long chain, unsaturated carbonyls, that might be produced during the process or storage. Furthermore, oxidation can result in premature ageing of wine and beer, which also a loss of flavor, color and aroma.
There are five mechanisms for the formation of volatile, long chain, unsaturated carbonyls: strecker degradation of amino acids, melanoidin mediated oxidation of higher alcohols, oxidative degradation of iso-alpha-acids, aldol condensation of short chain aldehydes and enzymatic or non-enzymatic oxidation of fatty acids. Various mechanism are most likely involved including the enzymatic degradation of fatty acids during malting and mashing followed by auto-oxidation of intermediates in the brewhouse to precursors which are oxidized in the package under the influence of free radical forms of oxygen (Huige, 64).
The browning of foods may contribute to both the color and the flavor of the food products. As stated before, they occur due to either enzymatic or nonenzymatic means. One of the types of browning reaction is Maillard reaction. Maillard browning reactions involve simple sugars and amino acids and simple peptides. They proceed during the kilning of malt, and during wort boiling. Maillard reactions have three basic phases. The initial reaction is the condensation of an amino acid with a simple sugar, which loses a molecule of water to form N-substituted aldosylamine. This is unstable and undergoes the famous Amadori rearrangement to form 1-amino-1-deoxy-2-ketoses (known as "ketosamines"), which can undergo complex subsequent dehydration, fission and polymerization reactions. The ketosamine products of the Amadori rearrangement can then react three ways in the second phase. One is simply further dehydration (loss of two water molecules) into reductones & dehydro reductones. These are essentially caramel products and in their reduced state are powerful antioxidants. Dark beer has about 5 times the reducing potential of pale beer. A second is the production of short chain hydrolyctic fission products. These then undergo the famous Strecker degradation with amino acids to aldehydes and by condensation to aldols. Negative aromas like 2 & 3-methyl-butanal and other aldehydes are also formed. This process can produce in the third phase, the favorable and important aroma of the hetrocyclic compounds. These can be pleasant flavors or burnt flavors. However, negative strecker aldehydes do not generally appear in finished beer in concentrations above their threshold level. Vigorous boiling and fermentation eliminate most of the more volatile strecker aldehydes. A third path is the schiff's base/furfural path. This involves the loss of 3 water molecules, then a reaction with amino acids and water. These also undergo aldol condensation and polymerize further into true melanoids. All these products react further with amino acids in the third phase to form the brown pigments and flavor active compounds collectively called melanoids. These can be off flavors (bitter, burnt), off aromas (burnt, onion, solvent, rancid, sweaty, cabbage) or positive flavors (malty, bread crust-like, caramel, coffee, roasted) and positive aromas (bready, cracker, fine malt).
Oxidation reaction can be reduced by several methods:
maintaining a high reducing potential through oxidation control in the
brewhouse, minimizing oxygen pickup by the product in cellar operation, during
packaging, and during product storage, minimizing oxygen radicals through
optimal use of endogenous and exogenous antioxidants and through minimizing copper
and iron pickup, and avoidance of high storage temperatures. Carbonyl compounds
formed or released during the storage of packaged beer are said to be the main
cause of oxidized flavor development. There are many carbonyls in beer
including additional aldehydes and ketones. Some of them are: Alkanels
(Pentanal, Hexanal, Heptanal, Octanal, Nonanal, Decanal), Alkenals (2-Hexenal,
2-Octenal, 2-nonenal, 2-Decenal), and Alkadenals (2,4-nonadienal,
2,6-nonadienal, 2,4-decadienal) (Huige 64-67).
Foam
The presence and characteristics of foam in beer and sparkling wine are important to the customer appeal of these products. Sparkling wines should have a foam of transient character, in which individual bubbles break quickly upon reaching the exterior surface (Senée 140). Beer drinkers prefer there to be a thick, stable head on their beer.
There are three basic attributes by which a foam can be characterized: formation, retention, and lacing. Formation is a measure of the actual quantity of foam that can be produced on the product surface. There should generally be little trouble producing an adequate foam, given the amounts of gas dissolved in beers. Retention is a measure of the ability of the foam to adopt a stable, polyhedral formation that allows for the foam to remain for an extended time. Lacing is a reflection of the foam’s ability to remain adhered to the walls of a clean glass, which consumers view as an indicator of foam quality. (Bamforth 48)
While the goals are quite different between brewer and winegrower, the mechanism that governs this phenomenon is the same for both products. Foam formation is fundamentally driven by micelle formation. As gas bubbles emerge from solution in the liquid they interact with amphiphilic glycoproteins. The hydrophobic protein end of the chain is drawn to the gas/liquid interface, forming a micellar sphere (Hough 139).

Figure 1
As the rigid bubble moves to the surface of the product it carries fluid with it. The properties of the hydrophilic carbohydrate chain greatly affect what happens to the liquid when it reaches the product surface. Very long chain carbohydrates can greatly increase the local viscosity at the bubble exterior. A higher viscosity allows for the liquid to stay entrained within the foam matrix, creating a moist foam with much the same flavor and the original beverage. However, if the carbohydrate chain does not create an adequately high viscosity at the surface the liquid simply runs off when the bubble reaches the surface of the product, resulting in a dry, undesirable foam (Hough 140). The high viscosity of the foam also allows for the foam to cling to the glass surface as the consumer drinks, resulting in the fine white rings that so many beer drinkers view as an indicator of foam quality (Bamforth 48).
Given this knowledge of the mechanism of beer and wine foam, information must be obtained about the specific substances that contribute to the phenomenon so that it may be adjusted as desired by the producer. It is clear that the relative hydrophobicity of the proteins present will determine how readily they form micellar spheres. This hydrophobicity can be measured by observing their binding behavior with hydrophobic resins. Those proteins that bind strongly to resins in experimental conditions have been shown to increase foam retention, as measured by Rudin head retention value (HRV) (Bamforth 49,51).
The effect of the hydrophilic, long chain portion of the glycoprotein is slightly less well understood. It is clear that an increase in the length of the chains surrounding the micellar sphere increases the local viscosity and encourages liquid retention within the foam. However, it is unclear as to whether the producer needs to simply increase the total level of large glycoproteins to increase foam retention — or decrease it to discourage foam retention.
Some studies indicate that rather than controlling the absolute level of large glycoproteins, the producer should be concerned with the proportion of large glycoproteins to small glycoproteins. If it is assumed that any beer will have sufficient glycoprotein content to create the needed micelles, then it becomes important to ensure that the proteins that do interact with the gas bubbles are those that will best allow for a desirable foam (Bamforth 53). Those hydrophobic molecules that don’t increase foam retention — oils, lipids, and small glycoproteins — will compete for positions on the bubble surface and detract from the ability of foam-enhancing glycoproteins to entrain liquid within the foam structure (Hough 140). By increasing the relative concentration of large proteins to small proteins, the brewer can ensure that only the foam-enhancing macromolecules will take part in these interactions (Bamforth 53).
Unfortunately, the amphiphilic nature of foam-enhancing glycoproteins makes them susceptible to loss during beer production. Additionally, some beers are brewed with additional sugar, rather than the malt from which many of the foam-enhancers originate, making them intrinsically deficient in the macromolecules needed for foam retention. Additives may be used at a latter stage of the brewing process to artificially account for these deficiencies. Hydrolysed albumen has been shown to be an effective source of large glycoproteins in this case.
Other chemicals have exhibited properties as foam-enhancers. These include hop bitters such as iso-α-acids, propylene glycol alginate, dextrins, β glucans, and melanoidins. The exact mechanisms by which these substances enhance foaming is not fully understood, but studies indicate that they may increase the bubble surface viscosity, and/or lead to cross-linking among the hydrophilic chains, leading to a more rigid bubble wall (Bamforth 52, Hough 140).
The use of different gases used to create the foam also has a notable effect on the properties of the foam. Carbon dioxide, present in nearly all beers and sparkling wines, creates relatively large gas bubbles and is readily soluble in liquid, which decrease the stability of the foam. Nitrogen generates smaller bubbles that increase the foam stability in three primary ways. First, the smaller bubbles rise through the liquid slower, giving them more opportunity to interact with the glycoproteins that lend rigidity to the foam. Second, the smaller bubbles are less prone to collapse by external stresses. Finally, the increased surface area of many small bubbles rather than a few large bubbles discourages fluid drainage from the foam (Bamforth 52, Hough 140). A number of pub-style beers employ various mechanisms for incorporating nitrogen into the beer as it is poured. The most notable of these are the Guinness Draught cans and bottles and their famous “floating widgets.”
The presence of ethanol in the product also effects the production of foam in beers and wines. Due to product specifications, this is not a property that can generally be adjusted by the brewer or winegrower, but an understanding of the causes help to present a more complete picture of foam formation. The absence of ethanol altogether results in a beer that cannot support a stable head. An increase of as little as 1% ethanol by volume has been shown to greatly increase the ability of beer to support foam. This increase is maintained through the range of most beer alcohol contents. However, at concentrations above 10% ethanol the beer begins to decrease its ability to support stable foam (Bamforth 53).
The actual causes for ethanol’s effect on foam stability are not fully understood, but various theories suggest that it plays a role through protein interactions (Senée 141), reduction of surface tension, and promotion of bubble-release by decreasing the solubility of carbon dioxide.
In addition to the aforementioned effects of short chain glycoproteins, other substances exist that can diminish the presence of foam on beer or sparkling wine. During the fermentation process of beer, antifoams are used to allow for more efficient use of equipment. These materials are generally removed during the filtration process before packaging, but inadequate filtration may leave antifoams in the product.
Much to the brewer’s frustration, the most influential foam inhibitors may be introduced at the point of distribution. Oily materials in the distribution equipment or glassware maybe lead to foam reduction. Even the detergents used to clean these oils can cause foam depletion when not adequately rinsed with clean water. Even the consumer may be guilty of sabotaging his own beer by introducing oils or lipids from food being consumed with the beer. These hydrophobic materials all have the effect of competing for position on the bubble surface without lending the advantages of the long hydrophilic tail.
Haze
Haze is the characteristic cloudiness that is often found in beer and wine. This is an undesirable property since detracts from both the taste and feel of the beverage as well as the visual character. Recently, there has been much effort to understand and reduce the problem.
Haze can be classified into two different categories, permanent haze and chill haze. As its name suggests, permanent haze cannot be eliminated. Chill haze on the other hand disappears when the beer or wine is warmed to temperatures above 20°C. Though the chill haze does not seem as severe of a problem, it is often a precursor to the permanent haze and should be taken every bit as seriously (Bamforth 81).
There are several different causes for the haze, both biological and non-biological. The biological haze is a result of a contamination of bacteria growth (Grafton). In the non-biological category there are actually numerous causes, though the most common by far is a result of the interactions between polyphenol and proteins (Bamforth 81).
Two systems of measurement for haze in beer have been developed, one by the European Brewery Convention (EBC) and the other by the American Society of Brewing Chemists (ASBC). The measurements are made by passing a beam of light with a wavelength of 580nm through the beer and observing the angle of scattering. Both systems have recipes for creating standard solutions that can then be compared to the haze in beer. For the EBC system, the standard solution has a value of 100 EBC units, and for the ASBC it is 1000 ASBC units. Though they are not directly comparable, the ratio is about 1 EBC unit to 69 ASBC units (Grafton).
A possible mechanism for the formation of the polyphenol-protein haze has been proposed by Siebert et al (81). In it, the polyphenol acts as a bridge between the protein molecules creating a large network. Each protein molecule is assumed to have a fixed number of sites for binding, and each polyphenol has a fixed number of ends for these sites. As one end of the phenol molecule comes in contact with a site on the protein molecule, it will bond. The other end will eventually encounter a different protein molecule and bond to one of its sites. If this process is able to continue and develop a large enough system, a visible haze will form.
One of the observations made by Siebert et al in their experiments was that the haze formation was at a maximum for a certain ratio of polyphenol concentration to protein concentration. Both reducing and increasing either substance, but not the other, decreased haze formation. This can be explained by the above model. When the phenol concentration is less than the key value, there are many more protein binding sites than phenol ends. The phenol will not be able to bridge enough protein and the network will be smaller. When the phenol concentration is higher than the respective protein concentration, there will be more phenol ends than protein binding sites. The chances that both ends of a phenol molecule will be able to attach to a protein are slim, and again, the haze-causing network will be smaller. Siebert et al have created an illustration of this as can be seen in figure 2 (81).
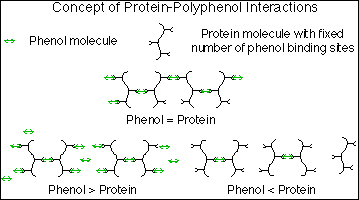
Fig. 2: Mechanism of Protein-Phenol Interactions
The polyphenols encompass many substances as the name means simply two or more phenol molecules. It has been found that there must be at least two hydroxyl groups on the aromatic ring for the polyphenol to be involved in haze formation. The more complex a polyphenol is, the greater its ability to form haze is. The most common complex polyphenols are catechin and tannins. These come mostly from the malt and hops in beer (Bamforth 83) and the skin and seeds of the grapes in wine (Sacuier 1045). One interesting note is that oxidation increases the ability of catechin to form haze (Bamforth 82). This is the reason for the decrease in head space when bottling.
There are many different proteins in beer and wine, however it is likely that only one or two are active in the haze forming process. For beer, these have been found to come from the protein barley hordein and are rich in proline (Siebert 1997). Recent studies have shown that it is the pathogenesis-related (PR) proteins, in particular thaumatin-like proteins and chitninases, that contribute to haze formation in wine (Waters 1637).
One apparent solution to the haze problem is to reduce the amount of haze forming protein. This seems to be effective for wine. Waters et al worked towards understanding the production of the proteins during grape growth with the hopes of controlling it by more carefully regulating environmental conditions. However, their experiments have shown that much of the protein development is inherent in the grapes and not simply because of stress during growth (1637). The use of manoproteins has been found to be beneficial for the average particle size of haze (Waters 3098). While the exact mechanism for this haze protection is not known, it is could fit into the haze forming mechanism of Siebert et al by interacting with the phenol and reducing the chance of it binding with the PR proteins to form the haze network.
There is some debate in beer, however about the value of these proteins. Some studies have shown that the haze forming protein is also foam forming, a desirable characteristic. Others claim to have found that there is a clear difference in the two (Bamforth 82). Unless there is found that, without any question, the proteins for the different functions are completely different, the removal of these proteins should not be used as a method for reducing haze.
A better approach for beer is to add polyinylpolypyrrolidone (PVPP) to adsorb the haze-forming polyphenols. There is some claim that the polyphenols have a contribution to the taste and feel of beer, however there is more evidence contrary to this than for it. In addition, PVPP may help with foam stability by removing the polyphenol, thus keeping it from precipitating out the protein involved in foam. Besides PVPP, there are many other approaches for eliminating haze in beer, including addition of Silica Hydrogels and Xerogels, Tannic acid, or Papain (Bamforth 84).
Filtration
Filtration of beer and wine is essential for quality control of a specific product. The process of filtration can be altered dependant on the desired properties. For example, filtering can control color, taste, haze and foam characteristics. All of these features of beer and wine are presented in the form of fairly large molecules and particles. Since these particles are large, they should be easy to filter out. Unfortunately, this is not the case.
For a desired product some types of colloids need to be filtered out, but others, which may be of larger or smaller size, need to remain in that specific product. A simple example of this is a problem with filtering beer. A desired attribute of beer is foam, and an undesired product of beer production is haze. Thus the filtration of beer must eliminate a majority of haze producing components, but must leave the foam producing molecules. This is a problem that can be avoided by using different methods and combinations of filtration. Some methods used are filtration and flocculation with filtration.
Tangential flow (cross-flow) membrane filtration has proven to be effective for treatment of brewing process residues containing yeast slurries (from fermentation tanks) and sediments (from conditioning tanks). By means of micro filtration it is possible to recover up to 65% bright beer from tank bottoms and thus increase brewing efficiency.
Microfiltration is the process of removing contaminants in
the 0.025 to 10.0µm range from fluids by passage through a micro porous medium
like a membrane filter. Although micron-sized particles can be removed by use
of depth materials such as those found in fibrous media, only a membrane
filter, having a precisely defined pore size, can ensure quantitative
retention. The retention boundary defined by a membrane filter can also be used
as an analytical tool to validate the integrity and efficiency of a system. For
example, in addition to clarifying or sterilizing filtration, fluids containing
bacteria can be filtered to trap microorganisms on the membrane surface for
subsequent culture and analysis. Membrane filters can be used for final
filtration or pre filtration. Depth filters are generally used in clarifying
applications where quantitative retention is not required, or as a pre filter
to prolong the life of a downstream membrane. Membrane and depth filters have
advantages and limitations, and can complement each other in a microfiltration
process system.
In all filtration applications, the permeability of a filter medium can be affected by the chemical, molecular or electrostatic properties of the filtrate. Since the microporous membranes can be operated in a tangential flow mode, it provides the ability to purify liquid such as wine or beer by removing particulate material, such as bacteria, yeast, and animal cells where large molecules must pall through the filter. The operating pressure for microfiltration is usually 0.1 – 3 bar.
Ultrafiltration is the process of separating extremely small particles and dissolved molecules from fluids. The primary basis for separation is molecular size, although a molecule's shape and charge can play a role. Ultrafiltration membranes retain materials of about 0.0005 microns (MW > 1000 Dalton) or larger, while salts and water will pass through. Colloidal and particulate matter can also be retained. Ultrafiltration membranes are usually operated in a tangential flow mode with feed material sweeping tangentially across the upstream surface of the membrane during filtration for maximum flux rates and filter life because ultrafiltration membranes can be repeatedly regenerated with strong cleaning agents. The operating pressure for ultrafiltration is 0.5-10 bar.
Reverse osmosis and nanofiltration are the processes of separating very low molecular weight molecules (typically < 1500 Daltons) from solvents (most often, water). The primary basis for separation is rejection of solutes by the membrane on the basis of size and charge. Unlike ultrafiltration membranes, reverse osmosis and nanofiltration membranes retain most salts as well as uncharged solutes. Nanofiltration membranes are a class of reverse osmosis membranes, which pass monovalent salts but retain polyvalent salts and uncharged solutes larger than 400 Daltons.
Flocculation is “a condition in which clays, polymers or other small charged particles become attached and form a fragile structure, a floc” ( http://www.glossary.oilfield.slb.com/Display.cfm?Term=flocculation). Since these particles attach, they increase their size and thus increase their filterability. Flocculation is mainly used to filter out the yeast and the haze in beer.
Beer and wine filtration involves different processes due to their different ingredients and desired characteristics. Beer requires the yeast and haze to be filtered out, while wine requires yeast, foam and flavanoid filtration. Wine is a slightly more delicate process due to the inclination of manufacturers and consumers to store wine for excessive periods of time.
Yeast is fairly easy to separate from beer and wine due to its tendency to flocculate and rise when all of the nutrients, sugars and other substances, are exhausted. Yeast can then be strained out by simple means. Unfortunately, in large-scale operation not all of the yeast at the bottom of the fermentor has a chance to rise and thus can cause fluctuations in the amount of yeast being passed through to another process. One way this can be solved is by using a greater amount of smaller fermentors (Hough 102).
Filtering beer haze is not as simple of a process. It involves flocculation between the haze particles and the absorbent or flocculant material. An absorbent must be chosen so that no desired component of the beer is filtered out. The way flocculation occurs is the absorbent has a charge, either positive or negative, which bonds to the charge of the haze. Since haze is a composite of two organic materials, which form a lattice and cannot form a lattice without one another, either one of the components can be filtered out. These two components are polyphenols and proteins. There are many different types of polyphenols and proteins, but there is a common bond between polyphenols: the hydroxyl group. The hydroxyl groups have a hydrogen atom which has a substantial charge associated with it. The hydrogens’ charges bond with the flocculants’ charges and thus form a floc (Siebert 75).
Many types of flocculants can be used with beer to produces these flocs of polyphenols including silica gel, bentonite, tannin, gelatin, polyvinylpyrrolidone (PVPP), et cetera. The more widely known absorbent is silica gel. It is widely used due to its cost, its ability to flocculate well and its tendency to not effect foam. Since silica only bonds to haze-active proteins, and not the foam proteins or the polyphenol, the silica seems to bond to the haze active proteins where the polyphenols bond. This mechanism can be shown by figure 3 (Siebert 73).
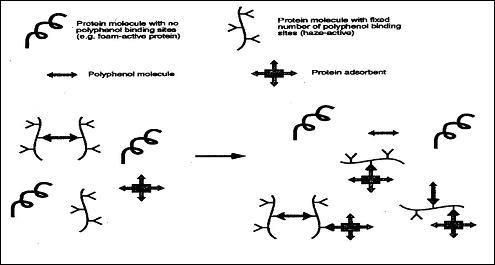
Fig.
3: Protein Adsorbent Fitting Into Haze Active Protein Molecule
PVPP works well with flocculating beer but is newer and more expensive. PVPP is a flocculant that attaches to the polyphenol part of the haze. Neither the haze-active proteins, nor the foam-active proteins are affected by PVPP. The mechanism for this flocculant is represented by figure 4 (Siebert, 75).
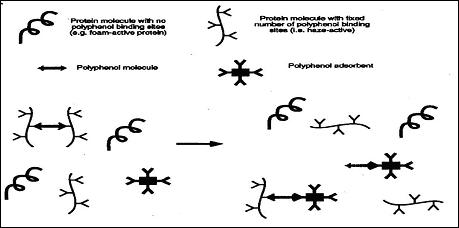
Fig. 4: Polyphenol Adsorbent Fitting into Polyphenol Molecule
Bentonite works well to flocculate the polyphenols, but also decreases the foam (Hough 142). Therefore bentonite cannot be used in conjunction with beer. Gelatin fining is a strong flocculate, due to its nature to bond with the haze-active proteins and no other component of the beer. Tannic acid fining is also a flocculate that bonds to polyphenols while slightly changing the protein concentration. Each of these flocculating materials’ performances are documented in figure 5 (Siebert 74).
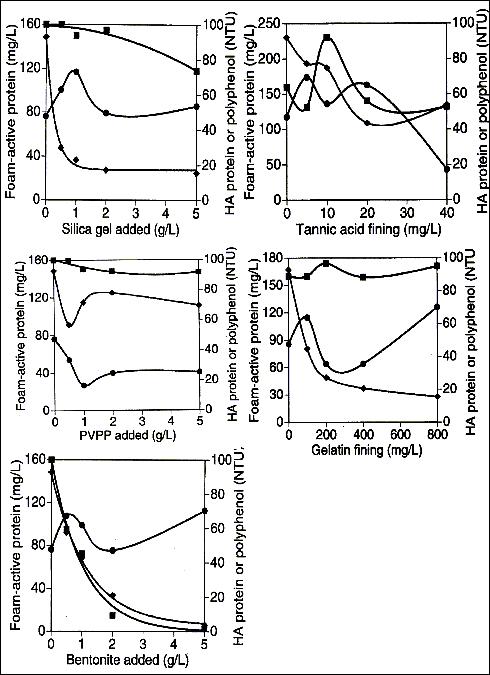
Fig 5: Performance of Protein Removal Additives
In figure 5, the (n) represents foam-active protein, the (l) represents haze-active polyphenols and the (♦) represents haze–active protein.
Filtration of the floc from the beer takes place in a cake filter containing either silica gel, or diatomaceous earth (DE or Kieselguhr) combined with silica gel. The diatomaceous earth filter simply traps the silica gel or other flocculate. During the time the flocculate is contained in the filter, it continues to absorb the polyphenols. The easiest way to run the flocculation/filtration process is at low temperatures. The haze is less soluable in low temperature solutions, and therefore it will more readily come out of solution and flocculate (Hough 142).
Wines, which require larger degrees of flavanoid and color filtration, are processed using gelatin fining, ultrafiltration, silica, et cetera. An example of a flavanoid is (www.chem.vt.edu/chem-dept/helm/3434WOOD/hmwrk/quercitin.gif):
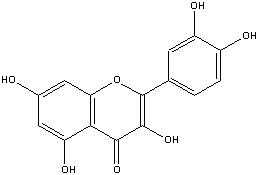
Fig 6: Quercitin
It is a flavanol, a type of flavanoid with hydroxyl groups attached, found in wine that causes bitterness and is desirable to filter out. Another type of flavanoid, which should be filtered out, is flavan-3-ol. These flavanoids can also polymerize.
Gelatin fining is the most common way to absorb these harsh flavanoids, but it absorbs particles that give the wine its fruity flavor and decrease its color. Ultrafiltration is a more customizable process that can be used to decrease the concentration of the unwanted flavanoids. Ultrafiltration incorporates membranes with semi-permeable characteristics, and separate particles according to size. Since the harsh flavors and colors of wine come from the largest flavanols and other molecules, filtration can work very easily for wine. Here is a chart labeling the approximate molecular weight for each component of wine (Shrikhande, 205):
|
Wine Component |
Molecular Weight |
|
Water |
18 |
|
Alcohol |
46 |
|
Reducing Sugars |
180 |
|
Acids |
150 |
|
Aroma Compounds |
<300 |
|
Simple Phenols |
<200 |
|
Cinnamic Acid Derivatives |
<400 |
|
Catechins |
300 |
|
Simple Flavanoids |
500 |
|
Procyanidins (complex flavanoids) |
600-2100 |
|
Coloring compounds |
>2000 |
Table 2: Wine
Composition as Related to Molecular Weight of Components
Knowing the composition and the molecular weight range for the unacceptable molecules, an approximate size of filter can be calculated. Ultrafiltration also benefits from the absence of the need for filter cleaning due to the unwanted, large molecules are flushed from the filter by a stream which is parallel to the membrane (Shrikhande 202).
Wine also contains polyphenols that can affect clarity of the wine. Generally bentonite is used to flocculate these particles. Bentonite is also good for flocculating foam. Since bentonite is a fairly cheap clay-like material, it is relatively inexpensive and easy to filter out later (Shrikhande197).
Since Gelatin is a
good flocculant, it can also be used to expunge the hazing material out of
solution. It is cheaper to use one flocculate and is simpler to filter out.
Even though this is true, most winemaking processes incorporate more than one
flocculant/fining medium. A common pairing is gelatin and bentonite.
Conclusion
Colloids effect beer and wine in many different aspects,
including processing, taste, color, texture, and overall consumer appeal. Due
to the history of brewing and wine-making, being that it has spanned over 2000
years, there has been ample time to get recipes correct and such. But, only
until recently the understanding of colloids has had a role in these processes.
Understanding colloids in alcoholic beverages yields a more efficient production
process, causing inexpensive brewing without sacrificing the consumers’
senses.
Sources Cited
Bamforth, Charles W.
“Beer Haze” Journal
of the American Society
of Brewing Chemists.
57:3 (1999): 81-90.
Bamforth, Charles W. “Beer Foams” Food Colloids. Cambridge: The Royal Society of Chemistry, 1989.
Bates, R.P. “Home Beer Making: Chemistry in the Kitchen.” Beer
and Wine Production:
Analysis, Characterization, and
Technological Advances. Washington
DC: American Chemical Society, 1993.
<http://www.chem.vt.edu/chem-dept/helm/ 3434WOOD/hmwrk/quercitin.gif> Last
viewed 6 April 2002.
<http://www.glossary.oilfield.slb.com/Display.cfm?Term=flocculation> Last viewed: 6
April 2002.
Grafton,
Gillian. Beer Hazes. Last viewed 7 April 2000.
<http://web.bham.ac.uk/GraftonG/haze.htm>.
Goldammer, Ted. http://www.beer-brewing.com. Apex Publisher. Last modified: 2002.
Hough, J.S., Biotechnology
of Malting and Brewing. New York: Cambridge University Press, 1985.
Huige, Nick J. “Progress in Beer Oxidation Control”. Beer and Wine Production. Ed.
Barry H. Gump. San Francisco: American Chemical Society, 1993.
Kirk, Raymond E., and Donald F. Othmer. Kirk-Othmer
Encyclopedia of Chemical
Technology 4th
Edition. Ed. Jacqueline I. Kroschwitz and Mary Howe-Grant. New York: John Wiley and Sons, 1998.
Supplement volume.
Millipore Corporation. http://www.millipore.com/catalogue.nsf/docs/C5981. Last
updated: 2001.
Nachel, Marty, and Steve Ettlinger. Beer for Dummies. Foster city, CA: IDG Books
Worldwide, Inc., 1997.
Nice, Karim. http://biz.howstuffworks.com/beer. Howstuffworks.com Inc. Last updated:
2002.
Saucier,
Cedric, Guy Bourgeois, Christiane Vitry, Didier Roux, and Yves Glories.
“Characterization of (+)-Catechin-Acetaldehyde Polymers: A
Model for Colloidal State of Wine Polyphenols”
J. Agric. Food Chem. 45
(1997): 1045-1049.
Senee, B, B. Robillard, and M. Vignes-Adler. “Foaming of Glycoprotein Alcoholic Solutions.” Food Emulsions and Foams: Interfaces, Interactions, and Stability. Cambridge, UK: The Royal Society of Chemistry, 1999.
Shrikhande, A.J. and S.A. Kupina “Ultrafiltration:A New Approach for Quality
Improvement of Pressed Wines.” Beer and Wine Production: Analysis, Characterization, and Technological Advances. Washington DC: American Chemical Society, 1993.
Siebert, Karl J., Aurea Carrasco, and Penelope Y. Lynn. “Formation of Protein-
Polyphenol Haze in Beverages.” J. Agric. Food Chem. 44 (1996): 1997-2005).
Siebert, K. J. and P.Y. Lynn. “Mechanisms of Beer Colloidal
Systems.” American
Society of Brewing Chemists. 55:2 (1997):73-78.
Siebert, Karl J., Nataliia V. Troukhanova, and Penelope Y. Lynn. “Nature of
Polyphenol-Protein Interactions.” J. Agric. Food Chem. 44 (1996): 80-85.
Ullmann, Fritz. Ullmann’s Encyclopedia of Industrial Chemistry. Ed. Fritz Ullmann.
New York: Wiley, 2002. Vol.A28.
Waters, Elizabeth J., Isabelle V.S. Dupin, Brett M. McKinnon, Corey Ryan, Muryel
Boulay, Andrew J. Markides, Graham P. Jones, and Patrick J Williams. “Saccharomyces cerevisiae: Mannoproteins That Protect Wine from Protein Haze: Their Release during Fermentation and Lees Contact and a Proposal for Their Mechanism.” J. Agric. Food Chem. 48 (2000): 3098-3105.
Waters, Elizabeth J., Kenneth F. Pocock, Yoji Hayasaka, and Michael G. McCarthy.
“Thaumatin-like Proteins and Chitinases, the Haze-Forming Proteins of Wine, Accumulate during Ripening of Grape (Vitis vinifera) Berries and Drought Stress Does Not Affect the Final Levels per Berry at Maturity.” J. Agric. Food Chem. 48 (2000): 1637-1643.