High Density Polyethylene
Up until the 1950’s the only type of polyethylene produced was low density
polyethylene. Low Density polyethylene was being produced at extremely high pressures. This high-pressure polymerization created polyethylene with many branches; the branches are created due to intermolecular and intramolecular chain transfer during polymerization. The mechanism for the polymerization of low-density polyethylene is free radical polymerization. The uses of low-density polyethylene are limited due to high number of branches. Because of the extreme pressure needed to create low density polyethylene and its limited uses, Karl Ziegler was trying to create polyethylene at atmospheric pressure. Karl Ziegler, a German scientist, made the greatest contribution to producing high-density polyethylene.
The difference in high-density polyethylene and low-density polyethylene is the degree of branching. The mechanical properties change drastically when comparing high-density polyethylene to low density polyethylene. In general, the degree of branching in polyethylene determines its mechanical properties. For example, high-density polyethylene is more crystalline than low-density polyethylene because it contains fewer branches. Unlike low-density polyethylene, Karl Ziegler created polyethylene with the use of a catalyst at atmospheric pressure. However, at first, this process did not go very smoothly. At first, Ziegler reacted Aluminum triethyl, a metal alkyl, with ethylene gas at atmospheric pressure. The reaction only yielded polyethylene with a molecular weight of 4,000. The reason for this is due to the displacement reaction where the aluminum-carbon bond displaces into a double bond.
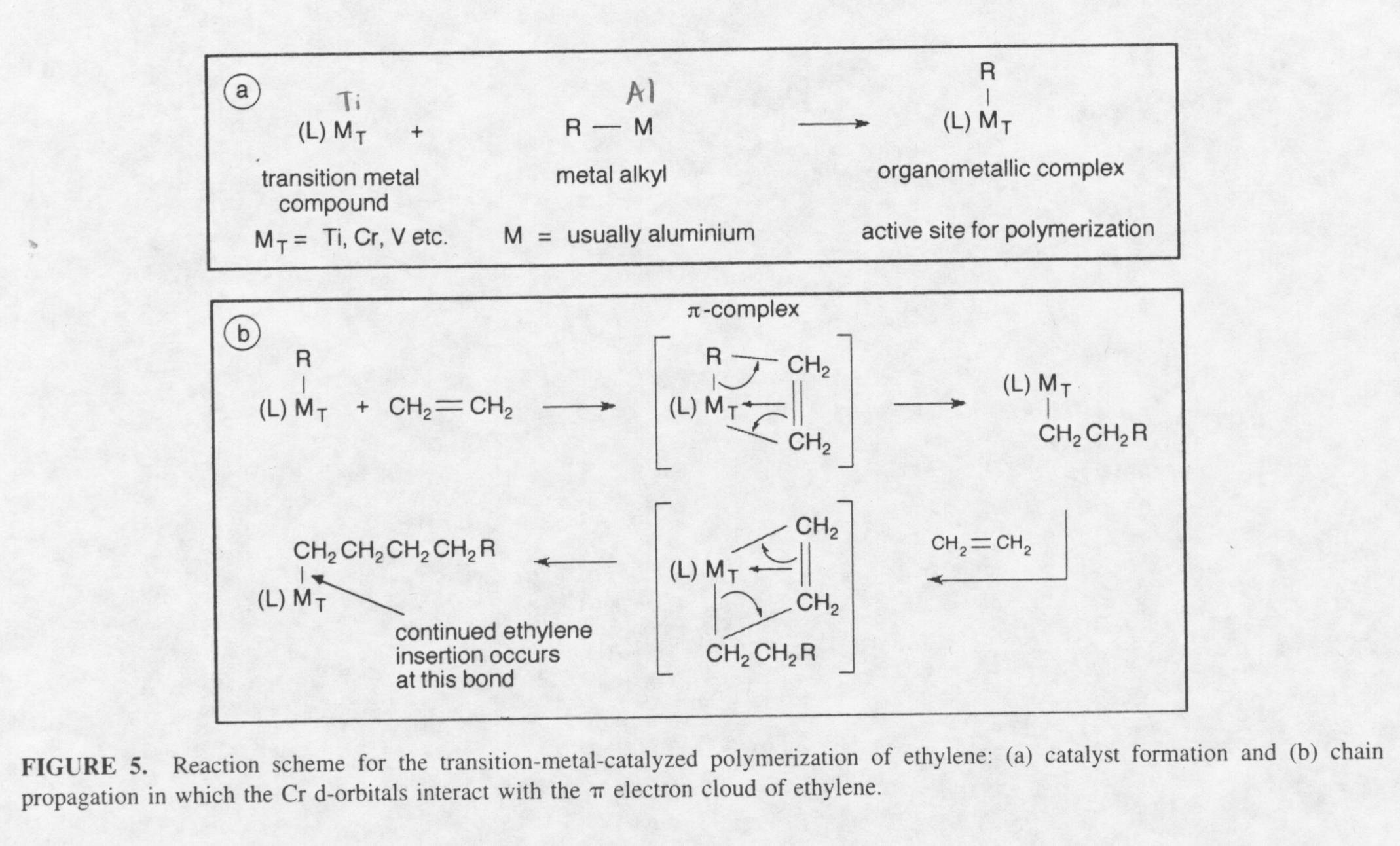
Ziegler realized the problem was due to the reactivity of aluminum triethyl, as shown in the reaction above. Because of this, Ziegler reacted aluminum triethyl, a metal alkyl, with titanium tetrachloride, an organometallic. He hoped the reaction of the two compounds would create an active site where polymerization would occur. When the two compounds were placed in a reactive vessel the precipitate titanium trichloride forms along with small amounts of unreacted aluminum triethyl. The titanium trichloride has a lower valence state than titanium tetrachloride, thus, making it more reactive than titanium tetrachloride in the presence of the monomer ethylene. When ethylene was introduced to the precipitate along with an inert solvent, polyethylene with a high molecular and very little branching was formed. This polymerization took place at atmospheric pressure and 100° Celsius.
How did this polymerization occur? The question is still debated today. The mechanism of the reaction to create high-density polyethylene is still not fully understood today. The question that persists is where the active site of the polymerization takes place. Today, the theory put forth by Natta is most credibly believed. Natta worked alongside Ziegler in a laboratory in Germany. He created polypropylene by reacting an organometallic with a metal alkyl to create an active site for polymerization. After creating the catalyst, Natta introduced propylene to create polypropylene. The catalyst created is very similar to the catalyst Ziegler created to produce high-density polyethylene. Because of this, the catalyst’s used today to create polyethylene and other types of polymers are referred to as the Ziegler-Natta catalyst. Natta explained the mechanism by explaining that the reactive site of the catalyst is the Ti-C bond and not the Al-C bond formed during the initiation step. The following reactions show the initiation step that creates the active site for polymerization, and the propagation step for the production of polyethylene:

The idea of the mechanism involves catalyst formation and chain propagation in which the Chromium d-orbitals interact with the p electron cloud of ethylene. The mechanism is classified as anionic polymerization or "living" polymerization. Living polymerization means the polymerization continues until the concentration of ethylene runs out. Because of this, the molecular weight of the polyethylene created can be extremely high. One way to control the molecular weight of high-density polyethylene created is through chain transfer reagents. Some typical chain transfer reactions are shown below:
Chain Transfer with monomer:
![]()
This reaction occurs with some heterogeneous Ziegler-Natta catalysts
Transfer with organometallic compounds:
![]()
This reaction leaves aluminum bound to the polymer chain. Exposure to moisture hydrolyzes the bond.
Chain transfer with hydrogen:
![]()
The reaction most important to controlling the molecular weight of high-density polyethylene created by the Ziegler Process is chain transfer with the introduction of hydrogen.
The production of high-density polyethylene from the Ziegler process is shown below:
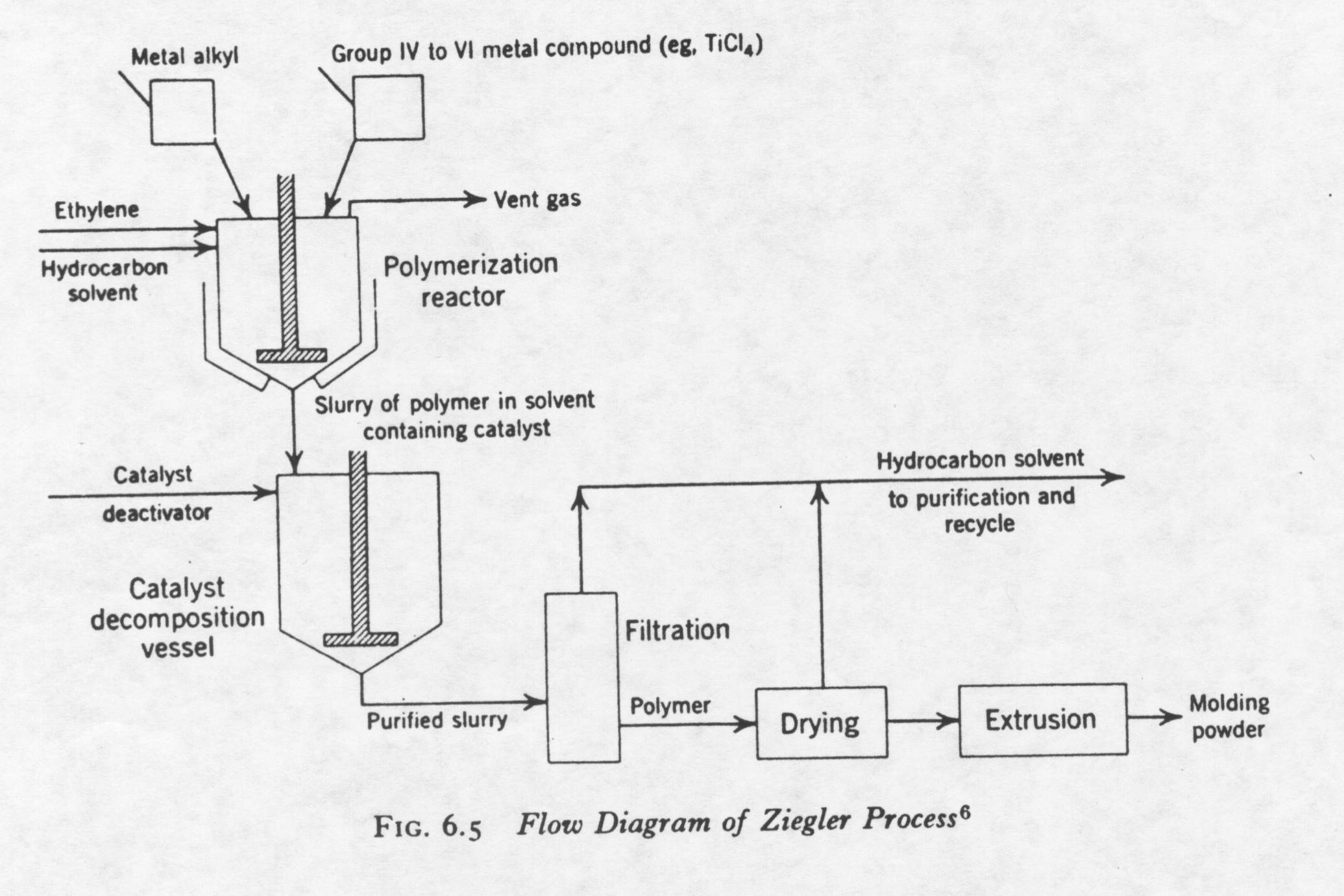
A brief description of the Ziegler process will be explained in the following paragraph. First, the organometallic compound (i.e. titanium tetrachloride) is reacted in a reaction vessel with a metal alkyl at a temperature between 100-130 degrees Celsius in the presence of a solvent. The pressure of the reaction vessel is between atmospheric and 20 atm. Ethylene is introduced into the reactor vessel in the gas phase. The boiling point of ethylene is approximately –100 degrees Celsius. The ethylene reacts with the active site of the catalyst to produce polyethylene. The solvent is used to dissipate heat. The solvent must not vaporize or react with any of the compounds in the reactor (inert solvent). The melting point of high-density polyethylene is approximately 130 degrees Celsius. Therefore, the polyethylene formed is in the solid phase. This type of polymerization is called slurry polymerization or suspension polymerization. The slurry solution is passed to a catalyst decomposition bed where the catalyst is deactivated. The catalyst is not completely used in the polymerization process. Catalyst decomposition is achieved with the addition of an alcohol. Polyethylene is then recovered with the extraction of the solvent, and a filtration and drying process. The polyethylene can then be processed and manufactured. The polyethylene created by the Ziegler process has a molecular weight 20,000 and 1.5 million. The molecular weight is controlled in a number of different ways: pressure of the reactor vessel (higher pressure, less branches), temperature in preparation of catalyst (too high of temperature deactivates catalyst), chain transfer reagents, and the ratio of Al/Ti catalyst added to reactor. The table below shows the weight average for different Al/Ti ratios for the Ziegler process at atmospheric pressure:
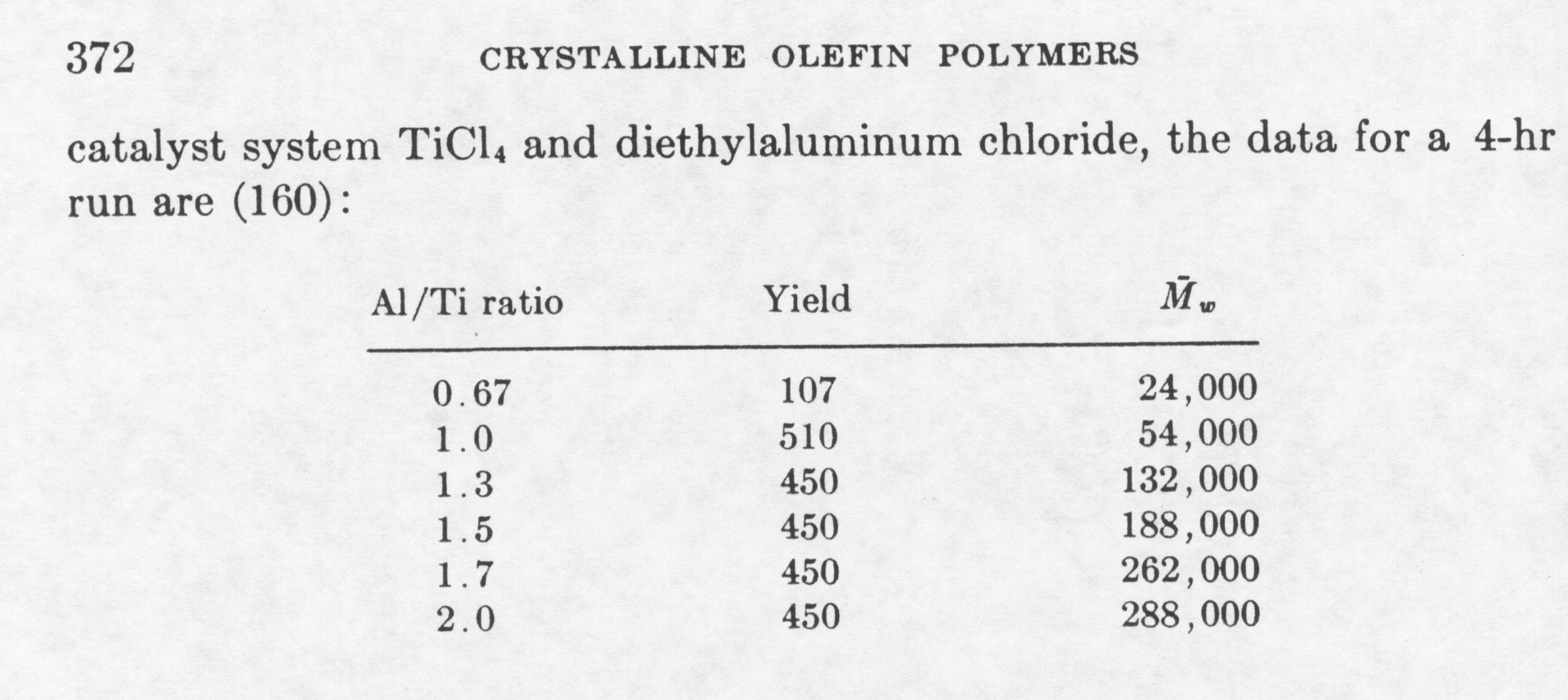
The results show the greatest yield is when the ratio is when the Al/Ti ratio is 0.9. The molecular weight continues to rise over an Al/Ti ratio of 2.0, however, the weight average remains constant. The Ziegler process produced a high-density polyethylene at pressures as low as atmospheric pressure. High-density polyethylene is a more durable polymer when compared to low-density polyethylene due to its lower degree of branching.
Phillips Process
The Phillips process is very similar to the Ziegler Process. The Phillips Process, the process commercialized by Phillips Petroleum Corporation in 1961, uses a catalyst to create an active site for polymerization. Historically, this was the first method used for commercial ethylene polymerization with the original Ziegler catalyst. At present, Phillips Petroleum utilizes a highly active catalyst, chromium oxide on a high-surface area silica, to produce high-density polyethylene. The active site for polymerization, Cr-C bond, is achieved by reacting the catalyst with an olefin. The olefin reduces the valence state of the transition-metal atoms, thus, making it more reactive. The reaction mechanism is similar to the mechanism explained for the Zeigler process. The mechanism is classified as anionic polymerization or "living" polymerization.
The difference in today’s process to produce high-density polyethylene versus the Ziegler process is a result of the catalyst used. The first generation Ziegler catalysts were not very active and had to be removed through a complex extraction process. An alcohol was added to deactivate the catalyst. Many of the polymer processes had in front of the main reactor a special reactor in which the catalyst preparation took place and the viscosity, morphology, control was a very important step. The use of a silica base eliminates this problem. The catalyst is very active and it no longer needs to be removed because all of the catalyst is reacted with the monomer ethylene. The active sites on the monomer are equally accessible to the monomer throughout the particle. Therefore, the polymer chains grow not only outwards but also inwards, causing the granule to expand progressively. The polymer particle will be a replica of the catalyst particle if the mechanical strength of the particle is high enough. Because of the complexity and importance of the silica base catalyst, the catalyst is often prepared in a separate production plant. The Phillips process is shown below:
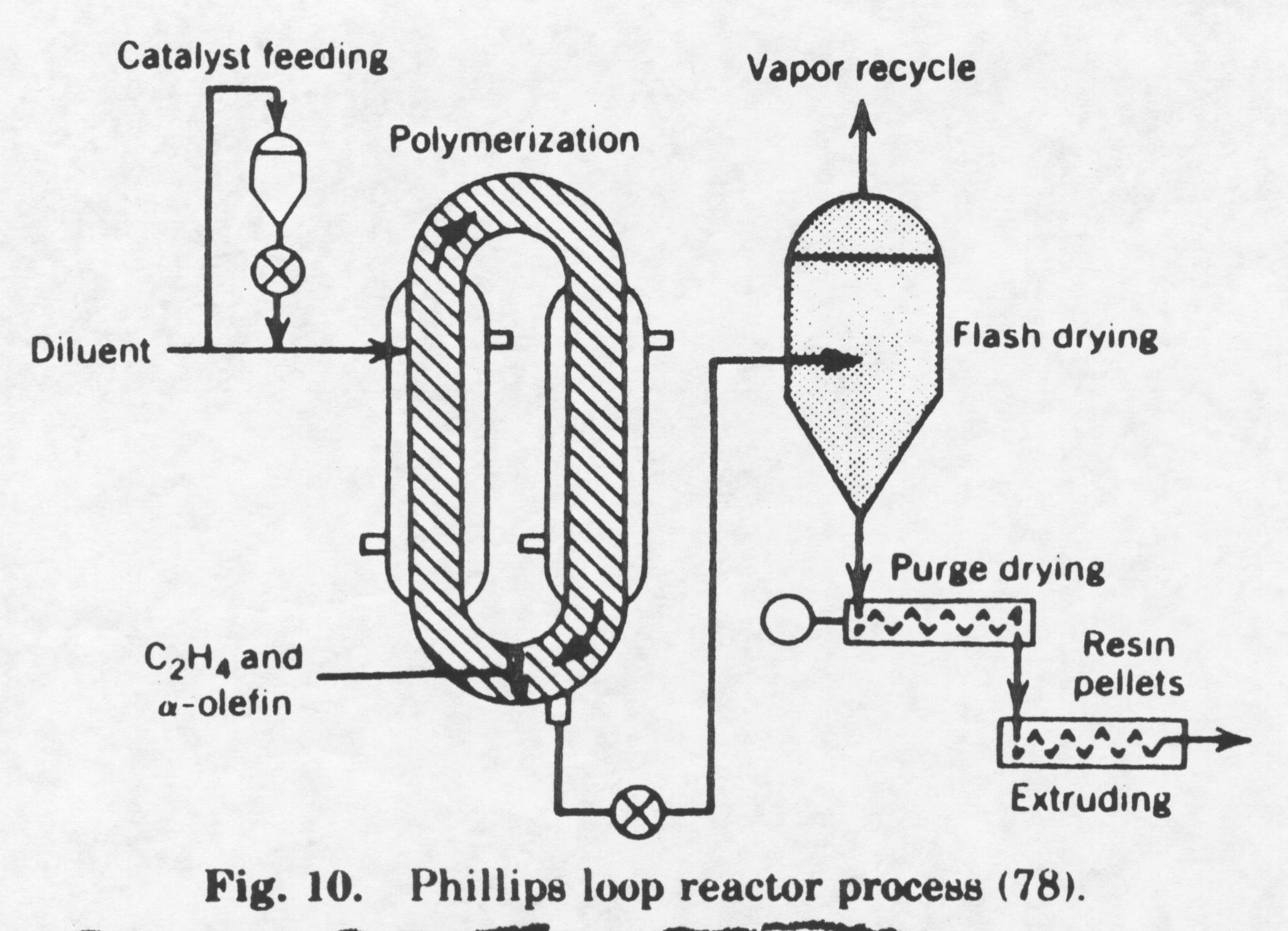
A brief explanation of the Phillips process is provided in the following paragraph. The process shown above represents Phillips Petroleum Co. suspension ethylene polymerization in 1961. The polymer particles are suspended in an inert hydrocarbon. The melting point of high-density polyethylene is approximately 135° Celsius. Therefore, slurry polymerization takes place at a temperature below 135° Celsius; the polymer formed is in the solid state. If the polymerization were to take place at a temperature greater than its melting temperature then the polymer formed would be in the liquid phase. The Phillips process takes place at a temperature between 85-110° Celsius. A loop reactor is used in a liquid-phase process. The catalyst and the inert solvent are introduced into the loop reactor where ethylene and an a -olefin are circulating. The inert solvent is used to dissipate heat as the reaction is highly exothermic. A cooling jacket is also used to dissipate heat. The active sites on the catalyst are equally accessible to the monomer throughout the particle. Therefore, the polymer chains grow not only outwards but also inwards, causing the granule to expand progressively. The reactor consists of a folded loop containing four long runs of pipe 1 m in diameter, connected by short horizontal lengths of 5m. The slurry of HDPE and catalyst particles circulates through the loop at a velocity between 5-12m/s. The reason for the high velocity is because at lower velocities the slurry will deposit on the walls of the reactor causing fouling. The concentration of polymer products in the slurry is 25% by weight. Ethylene, alpha olefin comonomer (if used), an inert solvent, and catalyst components are continuously charged into the reactor at a total pressure of 450 psig. The pressure is a lot higher than the pressure used to create high-density polyethylene by the Ziegler process. The high pressure creates HDPE polyethylene with fewer branches than the HDPE created by the Ziegler process. The HDPE created by the Phillips process typically has one ethyl branch per every 100 molecule chains while HDPE created by the Ziegler process has three ethyl branches per every 100 molecule chains. Because of this, the density of high-density polyethylene created by the Phillips process is higher. This has its advantages in processing. The HDPE created by the Phillips process is more crystalline and it is used to create more durable products. The polymer is concentrated in settling legs to about 60% by weight slurry and continuously removed. The solvent is recovered by hot flashing. The polymer is dried and pelletized. The conversion of ethylene to polyethylene is very high (95%-98%), eliminating ethylene recovery. The molecular weight of high-density polyethylene is again controlled by the temperature of catalyst preparation (too high of temperature increases spontaneous chain transfer, but increases the rate of reaction). The goal of the engineer is to find the temperature that optimizes the process. The molecular weight can be controlled by the addition of hydrogen into the reactor. Chain transfer will then occur. The following chain transfer reactions possibly could take place in the production of HDPE by the Phillips process:
Spontaneous Chain Transfer:
![]()
This is the principal chain-transfer reaction for the Phillips process with the use of a chromium catalyst. Its importance increases rapidly with increasing temperature and it provides easy means of controlling molecular weight.
Chain Transfer with hydrogen:
![]()
This type of chain transfer is not common for controlling the molecular weight for the Phillips process.
The Phillips process creates HDPE with fewer branches than the HDPE created by the Ziegler process. The use of a silica-based catalysts greatly reduces recovery and deactivation time. There are many different companies that produce HDPE today. They all use the idea of a silica-based catalyst and slurry polymerization. The reason why slurry polymerization is used is because solution polymerization has many disadvantages. The polymer created is in the liquid phase; only one phase is present. The polymer created is very viscous and for this reason a high molecular weight polyethylene can not be created by solution polymerization. Some companies use this process, but it is not very common. Another type of polymerization used to create HDPE is gas-phase polymerization. The process involves the reaction an a -olefin with an active catalyst, typically chromium based catalyst that is silica-supported, to create HDPE. This process is most commonly used to produce LLDPE and the molecular weight is controlled with the addition of the chain transfer agent hydrogen. The conversion rate for the production of polyethylene is very high for the Phillips process. The two processes explained above are only two ways to produce HDPE. The Ziegler process was the first method to create HDPE. The method of production of HDPE today using slurry polymerization is very similar to the Ziegler process. The only difference is that companies optimized the process to create a more efficient catalyst, a better way control the molecular weight, and a higher conversion rate of the monomer ethylene to polyethylene. The Phillips Petroleum Company is only company out of a number that optimized the Ziegler process to create HDPE in the most economical manner.