Colloidal and surface
phenomena project
“Hair conditioner”
Group members
Juan Carlos Alva Nieto
Ken-Tye Yong
Anthony Rivers
4 / 9 / 2002
Contents
Introduction
“What is hair conditioner?”……………………………………………………………….2
Hair conditioner as consumer product……………………………………………….……3
Hair chemistry
Human hair description……………………………………………………………….…...4
Macroemulsions and Microemulsions…………………………………………………….5
The Stability of emulsions………………………………………………………………...8
Foams
“What is Foams?”…………………………………………………………………………9
Interactions of surfactants and polymer with hair………………………………………..10
Adsorption………………………………………………………………………………..11
Types of hair conditioners
“Detanglers”……………………………………………………………………………...11
“Reconstructor”…………………………………………………………………………..14
Making procedure for the emulsion products……………………………………………16
Packaging………………………………………………………………………………...17
“Moisturizer”…………………………………………………………………………….18
Process control…………………………………………………………………………...20
References………………………………………………………………………………..20
Introduction
What is hair conditioner?
Hair conditioners are basically designed to restore hair to its natural state rather than produce an artificial effect. Mostly, the hair conditions have treatments of washing to dyeing or to sustain permanent waving hair [7]. Hair conditioner has also the ability to undo the damage by giving a better look and feel to the hair fibers. However, hair conditioners are not meant to repair damaged hair. Most hair conditioner is basically compositions containing cationic surfactants in combination with long chain fatty alcohol and other lipid components [1].
Modern hair conditioners are actually derived from old-age practices. For example, anointing the head with polar solvent, this gives protection to hair fiber during cleaning of hair. Hair conditioners were first formulated at the early 30, and it was based on self-emulsifying waxes, which then broke down into liquid form when it was massage to the hair. They were usually applied in conjunction with an electric heating cap. Early hair conditioners have only lubricating effect, and left a thin layer coating on the hair. At late 80’s further development have included liquid-foam form used immediately after a shampoo or for hair styling purpose. This liquid-foam form is often referring as hair “detanglers” that end “fly-away” hair and leave the hair to be soft, shiny and manageable.
Hair conditioner became popular and it is widely used today, many other brands and products of hair conditioner has appear in different form at the market. Even though, there are so much of hair conditioner products in the market, still it can be characterize into three main categories that is “moisturizers”, “reconstructors” and “detangles”.
Moisturizers are actually organic solvent concentrated with humectants.
Humectants are compounds that able to attract and hold moisture into the hair.
They may not necessarily contain botanicals or protein as compare to reconstructors’
hair conditioner [16]. As for
“reconstructors” hair conditioner, it contains
protein. For Hydrolized human hair keratin, protein will be the best source
since it has all 19 amino acids found in the hair. Human hair keratin protein
has a low molecular weight. This enables the proteins to penetrate the hair
shaft (the cortex) and gives better looking hair. A “reconstructors” also has
the ability to strengthen the hair [16].
Most “detanglers” are acidifiers
and have low pH. The function of “detanglers” is commonly used to close the
cuticle of the hair, which causes tangles. Some protection or
"shield" the hair shaft with surfactants and polymers. Some
detanglers are instant, some take 1-5 minutes to work.
As a summary, when consumer
continue to subject their hair to physical and chemical stress, hair
conditioners will definitely play an important role in the market.
Hair conditioner as consumer product
Hair conditioner became very popular around 90’s and it has an averaged
of 11 percent annual growth rate in gross rate. Nowadays, different brands hair
conditioners are sold in many places and the prices are reasonable since there
are too many competitors in the market.
As for safety concern aspect, most hair conditioners are fairly toxic
chemicals when swallowed. The oral lethal dose appears to vary from 100mg to
700mg since it depend on the body weight. Mostly hair conditioners have
quaternary ammonium compounds, and it serve as a cream rinses for hair. In high concentration of quaternary ammonium
compounds can injure the eye. Even at as low as 0.5 percent concentration,
quaternary ammonium compounds can do harm to the eyes such as conjunctivitis,
clouding of the cornea, and impairment of vision. Beside from quaternary
compounds, roughly of 2.5 percent of benzalkonium chloride or steralkonium
chloride was found in hair conditioners as well. At this percentage, swallowing
8 fluids-ounces of conditioners will be the lethal dose for a 40-pound child.
Therefore while selecting a hair conditioner it is important to know the ingredients
used in preparing for the hair conditioners and always keep it away from
children.
In most hair
conditioner, colorants is a must in the ingredients of formulating conditioner
since it is visual appealing and gives “better looking” to the conditioner.
However, color instability still might happen and it can be caused by many
reasons, such as the degradation of colorants, chemical interaction with
formula components, and ultraviolet radiation reactions [1]. Therefore, in
order to control the color stability in hair conditioner is to add ultraviolet
absorbers. By adding ultraviolet absorbers to the product it can absorb
degrading radiation and also inhibit prevention of product degradation.
Preservation of consumer products against microbial contamination is very important because contamination can lead to bad quality of product, furthermore the spread of disease. Thus, it is a must to preserve consumer products against microbial contamination at the time of manufacture and to ensure the products is able to preserved for a given time frame.
Hair chemistry
Human hair description
Human hair is a keratin-containing appendage that grows from large cavities or sacs called follicles. Hair follicles extend from the surface of the skin through the stratum corneum and the epidermis into the dermis.
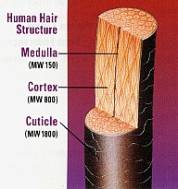
Morphologically , a fully formed hair fiber contains three and sometimes four different units or structures. At its surface, hair contains a thick protective covering consisting of layers of flat overlapping scale like structures called cuticle. The cuticle layers surround the cortex which contains the major part of the fiber mass. The cortex, the second unit consists of spindle-shaped cells that are aligned along the fiber axis. Cortical cells contain the fibrous proteins of hair. Thicker hair often one or more loosely packed porous regions called medulla, located near the center of the fiber. The fourth unit is the intercellular cement that glues or binds the cells together, forming the major pathway for diffusion into the fibers.
The human hair fiber can be divided into three distinct zones along its axis. The zone of biological, synthesis and orientation resides at and around the bulb of the hair. The next zone in an outward direction along the hair shaft is the zone of keratinization, where stability is built into the hair structure via the formation of cystine linkages, the third zone that eventually emerges through the skin surface is the region of the permanent hair fiber, the permanent hair fiber consists of dehydrated cornified cuticle, cortical, and sometimes medullary cells, and intercellular cement. Robbins (1) suggested that the diameter of human hair fibers varies from 15 to 100 mm.
Human hair is a complex tissue consisting of several morphological components (Figure 1), and each component consists of several different chemical species. It is an integrated system in terms of both its structure and its chemical and physical behavior wherein its components can act separately or as a unit.
|
The frictional behavior of hair is related primarily to the cuticle, but the softness of hair is determined by the cuticle, the cortex, and its intercellular components. Depending of its moisture content (up to 32% by weight) human hair consists of approximately 65 % to 95 % proteins. Its remaining constituents are water, lipids, pigment and trace elements. |
Figure (1). Morphological hair structure. |
Proteins are condensation polymer of amino acids, and the structures of those amino acids found in human hair are: Glycine, Alanine, Valine, Isoleucine, Leucine, Phenylalanine, Tyrosine, Lysine, Arginine, Histidine, Citrulline, Aspartic acid, Glutamic acid, Threonine, Serine, Cystine, Methionine, Cysteine, Cysteic acid, Proline and Tryptophan. Refer to Table (1) for a quantified description of the composition of the morphological components of hair:
|
Amino acid |
Cuticle |
Cortex |
Medulla |
|
Aspartic acid |
287 |
449 |
470 |
|
Threonine |
524 |
664 |
140 |
|
Serine |
1400 |
1077 |
270 |
|
Glutamic acid |
819 |
1011 |
2700 |
|
Proline |
994 |
667 |
160 |
|
Glycine |
611 |
485 |
300 |
|
Alanine |
--- |
374 |
400 |
|
Cystine |
2102 |
1461 |
Trace |
|
Valine |
6347 |
499 |
320 |
|
Methionine |
38 |
53 |
40 |
|
Isoleucine |
184 |
249 |
130 |
|
Leucine |
418 |
516 |
700 |
|
Tyrosine |
132 |
184 |
320 |
|
Phenylalanine |
91 |
142 |
--- |
|
Cysteic acid |
68 |
29 |
--- |
|
Lysine |
--- |
217 |
740 |
|
Histidine |
--- |
71 |
100 |
|
Arginine |
360 |
529 |
180 |
|
Ammonia |
--- |
--- |
700 |
Table (1). Amino acid composition of the different
morphological components of hair.
(Micrograms of amino acid per gram of dry hair)
Macroemulsions and Microemulsions
An emulsion may be defined as a mixture of particles of one liquid with
some second liquid, and, since almost invariably one of them is aqueous in
nature, the two common types of emulsions are: oil-in-water and water-in-oil.
That is, the term “oil” is used as a general word denoting the water-insoluble
fluid, and the term “water” is similarly used to denote the aqueous phase.
|
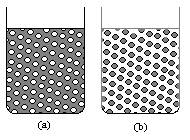
The abbreviations O/W and W/O are frequently used to denote the two above types of emulsions, respectively. These two types are illustrated in the Figure (2), It is clear that one phase (the “outer” phase) is continuous whereas the other (the “inner” phase) is not. |
Figure (2) Two types of emulsions: (a) Oil in Water O/W. (b) Water in Oil W/O |
Microemulsions are transparent or translucent single-phase systems of oil, water and amphiphile in which large swollen micelles are dispersed. It is well known that a larger amount of surfactant is usually required to form single-phase microemulsions compared with macroemulsions [2]. (In cosmetic applications, it is often very important to minimize the amount of surfactant for cost and safety reasons.)
Spontaneous formation, clear appearance, thermodynamic stability, and low viscosity are some characteristics of microemulsions that render these systems attractive and suitable for many industrial applications. The widespread use of and interest in microemulsions are based mainly on the high solubilization capacity for both hydrophilic and lipophilic compounds, on their large interfacial areas, and on the ultra low interfacial tensions achieved when they coexist with excess aqueous and oil phases [2].
In some applications, microemulsions and emulsions could be used. However, microemulsions have important advantages. Low energy input is required for their preparation (spontaneous formation) and stability. Their isotropic or clear appearance not only is an aesthetic property of interest for consumer products but also allows applications such as photochemical reactions, for which emulsions are unsuitable. For applications, which requiring high solubilization power, microemulsions are with any doubt superior than emulsions.
Many proteins can be solubilized in microemulsions based on apolar solvents, such as aliphatic hydrocarbons without denaturation of loss of function. This is remarkable because most proteins are sparingly soluble in apolar solvents and that transfer of proteins into these solvents, and that transfer of proteins into these solvents frequently results in irreversible denaturation and loss of biological activity.
Most hair conditioner are used today is consider as a microemulsion systems due to the extremely fine droplets of “oil” form in contrast to the ordinary macroemulsions. However, there is no clearly defined region and boundary between “microemulsion” and “macroemulsions”. But, it is generally agree that the lower limit of a “macroemulsion” is around 0.1 micrometer of size droplet. As for microemulsion is around quarter of visible light. For example, fragrance and flavor oils are used in preparing hair conditioner at relatively low concentrations. When fragrance oils is dissolved microemulsion system is form.
The Stability of emulsions
Proteins, glucosoides, lipoids, sterols, etc., although generally very water–soluble, are nonetheless frequently able to impart considerable stability to emulsions and foams. Saponin, albumin, pectin, gelatin, lecithin, and casein are among the natural substances possessing emulsion–stabilizing properties. (Berkman&Egloff -4)
In the foams as with the emulsions, it seems necessary to have some third surfactant present to insure stability, pure liquids foam slightly or not at all, and such bubbles as may be produced collapse quickly. Similarly, some of the methods for breaking emulsions, such as heating, freezing, or the addition of certain surfactants, may also be applied to foam prevention or elimination.
There are two problems of considerable interest in connection wit foams, but of much less importance in the case of emulsions, are the nature of the mechanical structure of the foam system and the mechanism of foam drainage. With respect to foam structure, there are two extreme situations. In one of them, the liquid phase is rather viscous and the foam consists of nearly spherical bubbles separated by rather thick liquid films. The other extreme situation contains mostly gas phase, and structure-wise, consists of gas cells separated by thin films, this due the low viscosity of the liquid.
Foams
What is Foams?
Foams can be considered as concentrated emulsions, having a das instead of a liquid as the dispersed component. On the other hand, the fact that the formation of an emulsion is often accompanied by the formation of foam indicates that, at least in these cases, foam systems originate under the same conditions as emulsion systems. Oils, which are readily emulsified in water, act as foam forming substances. Foam can be considered as a type of emulsion in which the inner phase is a gas, usually air. The inverse of foam however is an aerosol rather than another foam.
Foams as well as emulsions are three components systems, that is, they require the presence of an agent for their formation. Factors influencing the origin of foam systems and those influencing their ability to sustain themselves indicate another similarity between properties of emulsion and foam systems.
Foam is a formation in which microscopic and micronic and submicronic liquid layers separate ultramicroscopic gas bubbles. Therefore, foam is a union of laminae. The formation of foam occurs in the same manner as the formation of a single liquid lamina. Each film formed serves as a basis for the formation of other surface films; consequently, knowledge concerned with the formation of other surface films may account to some extent for a number of films, called in the aggregate “foam”.
The Stability of foams
There is no rigorous analysis possible of the interrelation of factors determining film stability and hence foam lifetimes. Qualitatively, the lifetime depends on the drainage rate, which in turns depends, in general, on some combination of fluid and film viscosities and on the elasticity of the film, which determines how extensively drainage must occur before rupture becomes probable. The factors (Adamson -6) affecting the stability of foams are a) low equilibrium surface tension, b) a moderate rate of attainment of equilibrium surface tension c) high surface viscosity as the most important properties leading to high foam stability.
It has already been made clear that foam-stabilizing agents will, in general, be surfactants, and most any substance capable of lowering the surface tension of the liquid phase will show some effectiveness in stabilizing foam. In addition, as was true for emulsions, solid particles forming a finite contact angle may also serve as foam-stabilizing agents.
Interactions
of surfactants and polymer with hair
Surfactants and polymer have become important components for hair care product over the past few decades. The more important uses of surfactants are as primary ingredients for hair conditioner, styling products and hair sprays. Interactions of surfactants and polymer with hair have been well studied over past few decade, especially in 90’s. Basically, it is convenient to describe three extreme types of bonds between surfactants and hair:
Primary valence bonds (ionic and
covalent bonds)
Polar interactions (Hydrogen
bonds)
Dispersion forces (van der waals
attractions)
Primary valence bonds include ionic and covalent bonds, which are the strongest binding forces. These bonds have energies of approximately 50 to 200 kcal/mol. Ionic bonds are extremely important to the interactions of cationic ingredients and hair. As for covalent bonds only involved between polymer and hair in certain polymerization reaction.
Hydrogen bonds are the most important polar interactions and are the next strongest binding forces. These bonds are meant to bind polymers that are containing polyalcohol or polyamide units, which also include polypeptides as well.
Dispersion forces or wan der waals attractions are relatively weak and are dipolar in nature. This is due to the electrons are in constant motion, at any instant at time the electrons distribution is probably distorted and creating a small dipole moment. These types of forces are short range and act only between the surfaces of molecules. Therefore, total strength of wan der waals bonding will increase when surface area increases.
Adsorption
The attachment of hair conditioning ingredients to hair fibers is fundamental to their action. The amount of sorption or uptake of an ingredient by hair from an aqueous solution is governed by its attraction or binding interactions to the keratin, its hydrophilicity or binding interactions to the aqueous phase, and the diffusibility of the ingredient into the hair.
For conditioning ingredients in shampoos and hair conditioners (1 -Robbins), have suggested that adsorption is more critical than absorption, because the conditioning ingredients are relatively large species.
The proteins in the hair conditioner (reconstructers) should have a low enough molecular weight to actually penetrate and reconstruct the hair cuticle, cortex and medulle. This product targets specific layers of the hair shaft working from the inside out, reconstructing, revitalizing and strengthening the hair.
Types of Hair conditioners
“Detanglers”
Purpose
and objective
As mention above “detanglers” has been widely used nowadays since there is a strong demand in the market. For hair to remain flexible and easier to comb a certain amount of moisture must be present within the hair shaft. Without this moisture, the hair becomes dry and brittle. These will results the hair to split and form tangles within the hair.
The basic purpose of using “detanglers” hair conditioner is to remove tangles from hair and gives straighten curly, and wavy hair. However, improvements have been made for the past decade. Therefore, many other beneficial functions have added in to “detanglers”. One of the crucial functions is to improve the texture of the hair and at the same time gives glass like shine to the hair. Another beneficial functions of using improved “detanglers” would be repairing and smoothen hair.
Ingredients
The common used ingredients to formulate “detanglers” are subdivide into five types are humectants, cationic surfactants, acidifiers, colorants and fragrance. Each of this has a unique role to play in formulating hair conditioner. In this part, we are going to discuss detail about these five categories.
In order to illustrate and explained on how this five types of ingredients work together, an example of common ingredients used to prepare “detanglers” hair conditioner is given as below:
Ingredients:
De-ionized water (94.6 % wt)
1-hexadecanol (co-surfactant) (2.5 % wt)
Glycerin (humectants) (0.5 % wt)
Stearalkonium chloride (cationic surfactant) (1.5 % wt)
Citrus acid (acidifiers) (0.4% wt)
Colorants (0.2 % wt)
Fragrance (oil) (0.3 % wt)
From the list of ingredients it is obvious that the weight percent for surfactants used in formulating hair conditioner is very little as compare to solvent. This is because excessive use of surfactants is usually undesirable not only from the viewpoint of cost but also from the safety concern and efficiency consideration. Many surfactants are irritating to the sebum when it is used in high concentration. Some surfactants can even promote penetration of other materials to the hair and increase the irritation potential on the surface of skin. Therefore, using too much of surfactants in formulating will not offer any beneficial effect towards the products.
As seen in the list two types of surfactants were used in the ingredients is 1-hexadecanol and Stearalkonium chloride. Stearalkonium chloride is belonging to the cationic surfactants family. Cationic surfactants are characterized by the fact that the hydrophobic surface-active grouping is positively charged. They are mainly used in deodorants, mouthwash, and hair care products. Stearalkonium chloride is also named quaternary ammonium salts. The application of quaternary ammonium compounds are based on the properties of positively changed nitrogen atom, which allowed the cationic group to be attach onto the negatively charged surfaces such as hair and skin. This physical reaction will neutralized the electrostatic charges on the hair and make hair easier to comb.
As for the co-surfactants 1-hexadecanol, which can be act as solubilizers in formulating hair conditioner microemulsion. Generally, it is always a rule of thumb to used a combination of two surfactants rather than one because this will gives better microemulsion form in the system. From thermodynamics point of view, the system will be much stable by using two surfactants since smaller droplet size may form.
In “detanglers” hair conditioner, glycerin serves as a type of alcohol that has a humectants action. Humectants are chemicals compounds, which are attracted to water. Humectants can hold and absorb moisture from the air. When humectants are applied to hair as a conditioner, glycerin will penetrate the cuticle and stabilize the moisture content within the hair shaft. The objective is to soften and swell dry brittle hair and results a shiny smooth looking hair.
Acidifiers are commonly found in most hair conditioner product. Acidifiers are able to dissolve the soap residue on the hair and at the same time it can eliminate greasy, sticking feeling that caused by the air pollution. When all these components are applied to hair, better manageable of hair can be achieved instantly.
Reconstructer
Reconstructer is a hair
conditioner, which rebuilds the hair. It deep penetrates in the hair and
represents an intense protein treatment that reconstructs dry, damaged and
chemically treated hair. Its ingredients penetrate deep into the layers of hair
to replenish the membranes lost in the hair shaft and on the scalp, or the cell
membrane complex.
The reconstructers are water-based products (microemulsions or foams). They are usually presented like foams, creams or mousses, which are rubbed into the hair and may then be rinsed off with water or allowed to remain on the hair for conditioning and styling benefits.
The reconstructer consists of an emulsion or foam. The main purpose of this product is to solubilize the proteins and to carry them to the hair surface. As it is discussed above the emulsions and the foams are good environment to solubilize proteins.
The reconstructer (hair conditioner) carries the amino acids / proteins until the surface of the hair, where it deposits them. These substances are the main components of the hair and consequently they are the re-building blocks that the hair will use to strength and reconstruct itself. Proteins are adsorbed onto the hair surface to reconstruct any possible hair damage. They act on the surface of the hair and penetrate it. Inside the hair, the proteins find the damaged area and stick on it. The amino acids and proteins rebuild the hair, and make it thicker and more manageable. (i.e. keratin, milk protein and wheat protein). On hair, the hydrolyzed or whole protein molecule exhibits extraordinary water binding and texturizing properties that impart better flexibility and body to hair and improve manageability. It also causes a reduction in split ends on damaged hair and induces superior gloss and easy combability.
Formulation of the product
Similar to the formulation of the other hair
conditioners, the reconstructers composition include: Anionic (emulsifiers),
lubricants additives, humectants, preservatives, water and fragance.
|
Anionic
emulsifiers |
Glycerol
stearate |
1.5
% Wt |
|
Cetyl
alcohol |
3
% Wt |
|
|
Lubricant
additives |
Stearyl
dimethicone |
4
% Wt |
|
Humectants |
Glycerin |
1.5
% Wt |
|
Propylene
glycol |
1.5
% Wt |
|
|
Preservatives |
Metyl
paraben |
0.30
% Wt |
|
Carrier |
Deionized
water |
87
% Wt |
|
Fragance |
|
Not
established. |
Table
(2). Typical hair conditioner
composition.
The emulsifiers are surface-active agents that promote the formation of intimate mixtures of immiscible liquids, preventing separation of the emulsion ingredients (water and emollients/humectants). (i.e. cetyl alcohol). The emulsifiers improve the formulation stability and the self-life. The emulsifier provides the creamy consistency of the conditioner and facilitates its application to the hair.
The emollients are fatty substances with lubricating action that have hydrating effects and make hair feel soft and smooth it. (i.e. lanolin –emollient from sheep’s wool/ moisture retention-).
The humectants are substances that increase moisture content and moisture retention of hair. They go directly to the hair, penetrate the hair shaft and promote the adhesion of the humidity on the hair surface. They make the hair to be more water retentive. (i.e. Glycerin)
The preservative are added to the
hair conditioner for the primary purpose of inhibiting the development of
microorganisms therein. (i.e Metyl
paraben).
The reconstructer’s main active agents are the proteins, amino acids, botanicals and amphoterics. All types of hydrolyzed proteins, such keratin, soy, yeast and wheat, chemically modified and free amino acids, such as cystine , aspartic acid and lauryl glutamate, and biological additives, such casein, beer, eggs, nettle extract, and horse chestnut extract have been formulated into this kind of hair conditioners.Refer to Table 3.
|
Hydrolyzed proteins |
Keratin, hydrogenated soy, yeast,
wheat germ glycerides. |
|
Amino acids |
Cystine, aspartic acid, lauryl
glutamate. |
|
Biological additives |
Casein, beer, eggs, nettle extract,
etc. |
|
Other |
Vitamins B-12, Inositol, Paba, and
Iron. |
Table (3), Most used rebuilding hair ingredients.
Making procedure for the emulsion
products
The following procedure is used to make oil-in-water (O/W) emulsions (most conditioners and conditioning shampoos) :
1) Dissolve the water-soluble ingredients in deionized water while stirring and heat if necessary.
2) If necessary, heat the oil-soluble components to melt the solids. These ingredients may be added together or separately. The order of addition is often critical. Before adding this components the deionized water and the ingredients solubilized in it should be heated to approximately 10 C above the melting point of the solids. After this add all the oil-soluble components while stirring.
3) Continue stirring for at least 10 to 15 minutes and then add the remaining water.
4) Cool, add preservative, fragrance and colors.
5) Adjust pH and then the viscosity.
The speed of agitation, type of mixer, rate of cooling, and the order of addition are all-important to produce consistent emulsion products that are stable and provide high performance.
Packaging
|
The reconstructers are disposed like
foams, creams or mousses. The commercial used package can vary from a bottle
(for the cream), an air-foam dispenser (finger pump foamer) Nowadays the finger pump foamer has been redesigned to improve its mechanical function, and it provides a precise mixture of liquid and air with just a single stroke of the smooth-action button. Based upon sophisticated valve technology, it is easy to use and extremely reliable. |
|
|
|
|
|
The Finger Pump provides instant foaming action. The pump can be fully filled and emptied completely due to the angular design of the dip tube, which allows the consumer to use the dispenser at any angle. The pump, which is made by Dutch company Airspray, requires no chemical propellant, The innovative 'Finger Pump Foamer' mechanical foam dispenser is now available in PET bottles. PET is the ideal bottle material to partner the Finger Pump Foamer, as it is lightweight with good mechanical strength. It also provides good neck definition, is compatible with a very wide range of products and gives excellent moisture and oxygen barrier performance. |
|||
The innovative dispenser system is easy to operate, a single push on the nozzle giving the ideal dose of high quality foam. An angled dip-tube inside the bottle ensures that the consumer can use the maximum amount of the product.
Moisturizers
Hair conditioners can accomplish many different jobs. They can moisturize, detangle, and
reconstruct hair. Each one of these
functions requires different types of chemicals to accomplish their job. The moisturizing part of hair conditioner
requires chemicals that will help make hair soft and manageable, make all the
hairs lay the same way, seals the cuticle layer of the hair and prevents split
ends. The chemicals that go into the
moisturing part of hair conditioners are as follows:
Deionized water
Hetoxide G-26
Methyl Paraben
Disodium EDTA
Hest CSO
Hetol G
Hetol CA
Hetamid MA
Glyceryl Stearate
Hetoxamate SA-100
Cocoa Butter
Hest MS
Hetsorb L-20
Propyl Paraben
Kathon CG
Glycerol
All of these chemicals contribute to the moisturizing aspect of hair conditioner. These chemicals can be categorized into different types of surfactants, anionic, cationic, nonionic, polymeric and ionic.
Anionic surfactants are surfactants that include, sulfates, sulfonates, carboxylates, phosphates, alkyl phosphates, phosphates, alkyl ethoxylated sulfates, dialkyl esters of sulfosuccinates.
Cationic surfactants are surfactants that include alkyl amines that have a chain length of C8 to C18.
Nonionic surfactants are surfactants that are stable over the entire pH range, and they are amphhiphilic, empirical HLB (hydrophilic-lipophilic balance) scale devised by Griffin.
Ionic surfactants are surfactants that are not affected by a change in temperature.
Polymeric surfactants are soluble polymers which has long hydrophobic groups, ( > C10 ). An example of a polymeric surfactant used in the hair moisturizer is glycerol.
All of these surfactants undergo micelle formation so that the surfactant chains minimize their interactions with water.
Some attributes that are affected by colloidal materials are the materials thickness, the ability to stay in the hair and its stability. Glycerol is added to hair conditioner so that it is thicker so that it can stay in the hair longer and do a better job. If hair conditioner was not as thick as it is it would run right out of the hair without doing anything. The proteins and other chemicals in the conditioner would not be able to interact with the hair before they are removed from the hair.
The major design considerations of hair conditioner is its ability to make hair soft and manageable, make the hairs all lay the same way, seal the cuticle layer of the hair and prevent split ends. Conditioners make hair softer and more manageable by filling in the holes in the hair that are left behind after you curl it, dye it or do other damaging things to it. Hair can become damaged just by going outside and doing things that you would do during an ordinary day. Hair conditioners make all the hairs lay the same way by making all of the charges of the hair neutral so that they do not repel each other and look like a mess. Conditioner seals the hair by leaving behind a protective layer that will help protect the hair from the elements. This makes the hair look and feel better. The protective layer that is left behind also prevents split ends from forming.
Process Control
Machinery:
The order in which ingredients are added is important to the production of hair conditioner. By mixing them in an order you can correct the amount of shear mixing so that you get a stable final viscosity. The basic ingredients in this process are quaterniery, hot fats, cold water, perfume and colour. The process to make conditioner is called the BRAN+LUEBBE. In this process all ingredients are controlled in proportion to each other. This system is shown in the diagram below (next page, also see power point)
Description:
Start with hot fats, quaterniery, hot water, being controlled by and Pentax in line dynamic controller, the Pentax mixer is usually fitted with a variable speed drive which enables amount of shear and final viscosity to be controlled. This mixture is then passed through an in-line heat exchanger, before perfume and colour are added.
References
1) Robbins, Physical and chemical behavior of hair.
2) Solans C. and Kunieda H. Industrial applications of microemulsions. Marcel Dekker
3) Johan Sjoblom, Emulsions and emulsions stability, Marcel Dekker.
4) Berkman and Egloff, Emulsions and Foams, Reinhold Publishing.
5) Feinberg Herbert, All about hair, Wallingford Press.
6) Adamson, Physical Chemistry of Surfaces, Interscience Publishers Inc.
7) Tom conry. “consumer’s guide to cosmetic” Anchor books, 1980
8) K.J.Saunders. “organic polymer chemistry” 2nd , chapman and hall
9) The colloidal domain Evans Wennerstrom, 2nd edition, wiley. Vch.
10) Interfacial forces in aqueous media. Carel J. Van Oss, Marcel dekker.
11) Principles of pratice of modern cosmetics.
12) Cosmetics science and technology.
13) CFTA Cosmetic Ingredients dictionary
14) Polymer handbook
15) The surfactants Virtual library
17) www.salonweb.com/pro/conditioner.htm
18) Consumer’s guide to cosmetics
19) www.geocities.com/hotsprings/4266/gloss.html
21) www.cfsan.fda.gov/~dms/cos-210.html
22) www.exploratium.edu/exploring/hair/hair_3.html
23) www.sunsethair.com/straighten.htm
24) www.purist.com.au/ingredients_no-propyl.htm
25) http://eudrams1.is.eudra.org/F3/inci/incifunc.htm
26) www.neopel.com/products/conditioners/info_conditioners.htm
27) Balsam, M.S. and Edward Sagarin, eds. Cosmetics: science and technology. John wiley and Sons: New York, 1974.
28) The Chemistry and Manufacture of cosmetics, D. Van Nostrand company, Inc, New York, 1962.
29) The Principles and Practices of Modern Cosmetics, Harry, Ralph G. Chemical Pulishing Co., Inc: New York, 1963.
30) Martin M. Rieger. “Surfactants in cosmetics”
31) Kozo shinoda. “solvent properties of surfactant solution”