CE457/527
(Photo courtesy of Reynolds metal company)
Submitted
to:
Dr.
P.Alexandirdis
Chemical Engineering
Department
State University of New York at Buffalo
Submitted
by:
Gregory Boehm
Vitor
Dasilva
Daniel
Fulcher
Jun
Wang
April 9, 2002
Table of Contents
Introduction
.3
The
Secrets of Human Hair Structure
..4
Definitions
/ Descriptions of Shampoo
5
Chemistry of Shampoo
8
Surfactants
Functions in Shampoo
... 10
Testing
Methods for Surfactants in Shampoo
15
Production Process
17
Marketing
considerations for Shampoo
18
Conclusions
19
References
. 20
Introduction
Colloid and surface phenomena is universally present in
almost every surface interaction involving fluids and solids there is. This phenomenon plays an important role in
the study and understanding of various topics ranging from globe related
occurrences to the processing and use of many industrial and ordinary household
products such as shampoo.
The focus of this report is to highlight the implications of
colloidal surface effects in the manufacture of different types of shampoo and
how this phenomena influence on their usage.
The interaction between these types of shampoo and the human hair will
also be studied.
Chemical and physical properties of both human hair and different types of shampoo must be identified in order to understand how colloid and surface phenomena takes place on the production and use of this every day product. Further sections of this report will define and discuss in detail such properties emphasizing on their colloidal behavior. Human hair structured will be explained in order to picture the media in which this behavior occurs. The composition of common shampoo types will be presented and their numerous agents identified. Different types of shampoo processing will also be discussed in detailed.
The secrets of Human Hair
Hair Structure
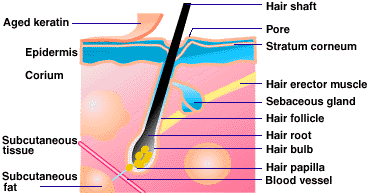
Hair is composed primarily of
proteins (88%), these proteins are of a hard fibrous type known as keratin. The
typical structure of hair is showing in Fig. 1
Fig. 1.
Structure of Hair [1]
Below the skin is the hair root, which is enclosed by a sack like structure called the hair follicle. Tiny blood vessels at the base of the follicle provide nourishment. A nearby gland secretes a mixture of fats called sebum, which keep the hair shiny and waterproof to some extern. Two sets of glands discharge secretions through the skin. Sebaceous glands arise from the walls of hair follicles and sweats glands embed in the subcutaneous layer which in the palms and soles. At the base of the follicle is the papilla; these cells play essential roles in regulating hair growth, hair cycle, and the size of the resultant hair. Surrounding the dermal papilla are epithelial keratinocytes and smaller number of melanocytes [2].
Hair is structured in three basic
layers. Packed dead cells surrounding
these structures are the cuticular layers of the hair. In the center of these structures lies the
medullar canal, which is actually apart of the excretory system and houses any
foreign debris, heavy metals, synthetics and medications that are thrown off by
the body and eventually released through the canal. The first layer is the cuticle.
A second, thicker layer is called the cortex and sometimes a third,
inner layer, called the medulla. The
cuticle is the outer layer of protective scales. The cortex provides
strength to the hair shaft, and determines the color and texture of hair.
The medulla is present only in thick, large hairs. [3]
Hair Chemistry
[1]
When the hair is in its normal unstretched state, it is referred to as A of alpha kertin. The original configuration of the hair is held in place by the bonding found in the cortex layers of the hair. There are four types bond.
The Hydrogen Bond
This bond is located between the coils of the alpha helix and is responsible for the ability of the hair to be stretched elasticity and return back to its original shape. These bonds are responsible for approximately 35% of the strength of the hair and 50% of the hairs elasticity.
The Salt Bond
The salt bond is also an ionic
(electrolytically controlled) bond formed by the electron transfer from the
side chain of a basic amino group to the side chain of an acidic amino acid. It
is responsible for approximately 35% of the strength of the hair and 50% of the
hairs elasticity.
The Cystine Bond
The
cystine bond also known as the disulfide bond, sulfur bond, or just S bond is formed
by cross-links between cystine residues of the main polypeptide chains. This
bond is perpendicular to the axis of the hair and between the polypeptide
chains. It is responsible for the hairs toughness or abrasion resistance.
The Sugar Bond
The sugar bond is formed between the
side chain of an amino acid having an OH group and an acidic amino group. It
gives the hair toughness but little strength (5%).
Hair Life Cycle [1, 3]
Hair is actually dead material when it
leaves its root. The root of a hair
fiber sticks in a bag in the skin. The fiber is pushed out of this bag about
0.35mm per day, making an average growth rate of 1cm, or half of an inch, per
month. The growth rate is however very much related to an individuals age, diet
and etc. Healthy hair has an average
lifetime of 2-6 years. After a rest
period of three months the single hair falls out, and a new fiber starts to
grow out of the bag. The lifetime also
depends on circumstances and each person.
The lifetime of hair is responsible for the maximum of hair length one
can have.
There are three phases in the hair life cycle:
Active growth phase, or anagen phase: the hair root produces the cells that
form the living part of the hair. This pushes the cells that already exist up
and out from the follicle.
Transition phase, or catagen phase: New cells are not created at this stage.
Instead, the hair follicle actually shrinks about 82%;
Resting phase, or telogen phase: The protein hair strand remains
connected to the hair follicle, but it doesnt grow. After five or six weeks,
the dermal papilla reconnects to the base of the hair follicle and the
bloodstream. The hair reenters the active growth phase.
Definitions/Descriptions of Shampoo
What is Shampoo[4]
In essence, shampoos are simply
detergents. They are a different type
of cleaning media than ordinary laundry or hand detergents because of their
application to different types of hair. Shampoos are used to remove excess oil,
dirt and skin debris from the hair known as sebum. A good shampoo will perform this function while leaving the hair
manageable. These products should
possess rich foaming action and rinse out easily. Various forms of shampoos are available, from clear liquids to
opaque pastes.
The primary ingredient of a shampoo is the detergent, either
from an organic soap or a synthetic. Vegetable oil soaps, alkyl benzene
sulfonates, sodium or triethanolamine alkyl sulfates, sulfated monoglycerides,
sulfated oils and nonionics are typical.
The concentration used varies with the individual detergent and the
shampoo type and will vary from about 10% to 50%. Shampoos usually include modifying agents such as opacifiers,
clarifying agents, antifreezes, conditioning and finishing agents, sequestrants,
thickening agents, proteins, foam builders, and antidandruff agents. The use level of these modifiers is usually
about 1% to 10%.
Functions of Shampoo
Depending on their functions,
shampoos are used as cleaning agents for cosmetic purposes, antidandruff
agents, antiseborrhoeic agents and keratolytic agents. [5]
As cleaning agents:
these shampoos should be mild, effective, without causing irritation and should
be used daily or on alternate days as needed. They remove dust and excess oil
from the hair.
As antidandruff agents:
these treat dandruff due to fungi like pityriasis versicolor. Rapidly relieves
scaling and pruritis which are associated with fungal infections.
As antiseborrhoeic agents:
they have cytostatic effect on cells of the epidermis and follicular
epithelium, thus reducing corneocyte production.
As keratolytic agents:
they remove ointment, pastes, which are used in the treatment of psoriasis.
They also remove hard scales from the scalp.
Key features of
different shampoos are showed in Table 1.[6]
Table1. Key Features of Shampoos
|
Type of Shampoo |
Key Features |
|
Clarifying Shampoo |
Contain heavy
duty surfactants. Used to deep clean hair and remove the gunky build up of
conditioners, sprays, and gels. |
|
Volumizing Shampoo |
Add body to limp
hair. Contain proteins that bond to hair and pump it up |
|
Moisturizing
shampoo |
Best choice for
dry, flyaway hair, make split ends look better, pull moisture onto hair to
keep it from getting too dry. |
|
Revitalizing
Shampoo |
Made for color treated,
permed, and damaged hair. Use as a gentler cleanser, protect color from
fading. |
|
Dandruff Shampoo |
Contain medication
that loosens and rinses away those annoying flakes. |
|
2-in 1 Shampoo |
With conditioner,
save time. |
|
Swimmers Shampoo |
Remove chlorine and
other minerals. |
Shampoo Design Consideration Factors
The first step to understanding the
chemical interactions that are present at a colloid level in a typical shampoo
is to first understand the parts making up the entire mixture. Shampoos rely on a selection of species,
which are included in the design to further a specific design goal. Within each of these categories is room for
selection to tailor a product more closely towards the intended audience.
It is important to design a shampoo
with its outcome clearly in view.
Desirable qualities for a shampoo are Lathering in hard or soft water,
easily and completely removable lather, without leaving a residue. Safe for repeated use, non-irritating,
chemically and physically stable, and not damaging to the eyes.[13]
Cleaning the hair is the primary
purpose of any shampoo product. This
rudimentary practical function is what separates shampoos from the host of
products, which are designed to increase the ability of hair to be styled or
otherwise maintained. Therefore one of
the first steps to be undertaken is the selection of a proper detergent for the
shampoo. Building foam is also important to the consumer, who without detailed
information about the workings of a surfactant assumes that the more foam that
is created the better the shampoo is working.
Working the lather into the hair is also used as a marketing tool for
shampoos. Most of the surfactants used
for human hair are slightly acidic.
Some surfactants with caustic properties can be used like various
chlorine salts. These alkaline
surfactants are typically more irritating to the scalp over prolonged periods
then their slightly acidic counterparts like sodium lauryl sulfate, and other
chains between 12 and 14 carbon atoms long with a hydrophilic head, empirical
experience has lead to the choice of carbon chains of this length[14]. Solubility of the surfactant is sometimes an
issue when the appearance of the shampoo is taken into account. If a clear shampoo is desired then choosing
a surfactant or blend of surfactants with a high solubility in water is
essential. Examples of these are fatty alcohols, and stearate soaps.
Thickness of the shampoo solution is an
important consumer design feature.
Shampoo is expected to behave in a certain manner when poured out of the
bottle. Viscosity greater then water
makes it easier to pour the correct amount of shampoo. It also helps it to stay in your hair when
it is being used so it does not come out before the application is completed. [14]
For the shampoos, which have a
chance of precipitating earth, metals formally attached to the surfactants
small amounts of chemicals to contain them are often added. These are termed sequesterants, and are
often used in the medical field to treat metal poisoning. Tetrasodium diethanolamine is an example of
one these compounds, they contain the loose metal ions and maintain the systems
integrity.
Shampoo is expected by the consumer
to have a long shelf life and preservatives are added to the mixture to
maintain this. Two common additives for
human shampoos are methyl paraben, and propyl paraben. These prevent microbial infestation of the
shampoo medium, which is otherwise so common in a warm moist environment like a
bathroom.
Other ingredients can be added as
well. Shampoo is an excellent way to
treat dandruff since the product is already in direct contact with the affected
area. Medication such as antimicrobial
agents like salicylic acid and cadmium sulfide. More recent has been the use of selenium sulfide. Fragrances can also be included in
shampoo. The limit on these additives
is largely determined by what the public will buy. In the same manner common color additives are also used to give
the shampoo a marketable appearance. [15,16]
Chemistry of Shampoo
General Composition of Shampoo
Shampoos are combinations of many
chemicals and water, the general compositions of shampoos are:[7]
- Cleaning agents: the prime ingredients in all shampoos
are substances called surfactants. Responsible for cleaning action and
laterring properties, they largely determine the hairs condition after
shampooing.
- Modifying agents: Shampoos contain
far more components other than surfactants. There are thickeners (xanthan
gum), preservatives (parabens), emulsifiers (glycol distearate), color
additives and foam boosters (cocamide monoethanolamine). Some shampoos
also include panthenol, which can diffuse into the hair shaft and bind to
proteins, strengthening their structure. Humectants, which help to retain
moisture, also are added. Ethyl alcohol, isopropyl alcohol and sodium
xylene sulfonate can be used to maintain clarity in shampoo.
- PH adjuster: In healthy hair, the
cuticle consists of translucent cells overlapping like shingles on a roof.
In damaged hair, these shingles are more open and ragged. As the rough adjacent hairs rub against
each other, transfer of electrons can produce a static electrical charge. The result is the dreaded affliction of
flyaway hair. Ideally, a shampoo
should smooth down the cuticle and cover it with a clean coating of a
sebum-like material. The smoothing
effect is readily achieved by controlling the shampoo's acidity. All shampoos, whether they make the
claim or not, are pH balanced. The
proper pH range is maintained by addition of buffering agents, such as
citric acid.
- Fragrance: Fragrance oils are added so that hair
is left smelling fresh, which attracts consumers.
Good and Bad Ingredients in Shampoo
In
shampoo, some chemical ingredients are rarely degradable or non degradable,
thus bad for environment, some ingredients are harsh for eye and skin, and some
ingredients are necessaries for cleaning and conditioning. As showing Table 2,
there is general guide to shampoo ingredients.
Table 2. The street Cents Guide to Shampoo Ingredients [8]
|
Good
Ingredients |
||
|
What
it is |
What
it does |
What
it's called |
|
Gentle
Surfactants |
Cleans
your hair |
sodium
laureth sulfate and ammonium laureth sulfate |
|
Silicone |
Conditions |
Dimethicone,
cyclomethicone |
|
Quarternary
Ammonium Compounds |
helps
create manageable hair |
Guar
hyroxypropyltrimonium chloride, dicetyldimonium chloride, dihyrodenated
tallow benqylmonium chloride, quaternium 18, stearalkonium chloride. |
Continue Table 2.
|
Panthenol |
adds lustre, movement, and
keeps in moisture |
Panthenol |
|
Proteins |
good conditioners, but
might just wash out |
collagen, elastin |
|
Humectants |
condition and keep
moisture in, but are water-soluble and might just get washed away |
glycerin, sorbitol,
glycols, propylene glycol |
|
Shampoo fillers |
||
|
What it is |
What it does |
What it's called |
|
Water |
a large part of all
shampoos |
water, aqua |
|
Preservatives |
keep out contamination |
Methylparaben,
propylparaben, phenoxyethanol, DMDM hydantoin, 2-bromo-2-nitropropate-1,
3-diol, imidazolidinyl urea |
|
Thickeners |
make shampoo thicker |
cetyl alcohol, stearyl
alcohol, hydrogenated lanolin, polyethylene glycol (PEG), glycol stearate,
palmitic acid |
|
Citric Acid |
keeps the pH level of the
shampoo balanced |
citric acid |
|
Foam boosters |
make more lather |
cocamide MEA, lauramide
MEA, lauric DEA, lauramine oxide, cocamidopropyl hydroxysultaine, polysorbate
20 |
|
Harsh Ingredients |
||
|
What it is |
What it does |
What it's called |
|
Surfactants that are harsh |
will make matters worse if
you have dry scalp or hair. |
Sodium lauryl sulfate,
alkyl sodium sulpgate, and sodium oelfin sulfate, TEA-lauryl sulfate |
|
Some "natural"
ingredients or essential oils |
May cause skin sensitivity
on your scalp or sun sensitivity |
almond extract, allspice,
angelica, arnica, balm mint oil, balsam, basil, bergamot, chamomile,
cinnamon, citrus, clove, |
Typical Surfactants for Common Shampoo
The major types of surfactants are
anionic, cationic, nonionic, and amphoteric. Ions are molecules that have small
electrical charges that may be positive or negative. Opposite charges attract
and similar charges repel. Surfactants with a negative charge are called
anionic. A surfactant with a positive charge is cationic.[9]
Anionic Surfactants:
Anionic surfactants carry a negative
charge when ionized. It provides a lot of the lather and detergency in the
shampoo. Because of their excellent cleanings, foaming, and solubility
properties. The most commonly used anionic are sodium laureth sulphate and
sodium lauryl sulphate. Usually using a primary fatty alcohol and treating it
with oleum, chlorosulfonic acid, or sulfuric acid make them. Sodium, ammonium,
and triethanolammonium (TEA) lauryl sulfates are often found in shampoos. [10,11]A
major disadvantage is that they can be harsh and irritating to the scalp. Frequently, other surfactants and
ingredients are added to reduce skin irritation.[9]
Cationic surfactants:
Cationic surfactants carry positive charge when ionized. They are used less frequently due to their dangerous threat to eyes if used in large quantities. The gentleness of your shampoo depends on the surfactant found in its ingredients. Cationic molecules have the ability to cling to wet surfaces by static attraction. Consequently they are not easily removed during the rinsing process and form the basis of conditioning. Polyquarternium-10 is one of the most common cationic conditioners. It is based on a cellulose polymer that is then quaternaries to give the desired properties.[10,11]
Nonionic surfactants:
Nonionic surfactant has no charge to
the molecule, it isnt used as a cleaning agent, but are often used in
combination with the primary cleanser to change or modify its actions, they
aid in solubility, modifying foam, and in some instances conditioning. They can
strip the hair and lead to scalp irritation due to excessive defatting. These
include laureth-3 or 4, cocamide DEA or coco glucosides.[10,11]
Amphoteric surfactants:
Amphoteric surfactant carries both positive and negative charges when ionized. They are very useful for decreasing the irritancy of a formulation while increasing the active contents level of the product and quality of the lather produced. Each amphoteric surfactant has cationic and anionic charge groups, positive and negative. Most amphoteric shampoo surfactants are used in baby shampoos, because they are gentle and wont burn the eyes. By far the most used is cocamido propyl betaine, or occasionally cocamido betaine. [10,11]
Surfactant Functions in Shampoo
Function of Different Surfactants
Surfactants will influence six
essential attributes of shampoo: cleansing, foam, condition, viscosity and
aesthetic appeal combined with safety and mildness in use.
Cleansing:[12]
Cleansing is a function of the
primary surfactant. To be an effective cleansing agent the surfactant system
must work quickly at a relatively low temperature. It must be effective in hard
and soft water, be able to remove lipids and other soils and residues left
after previous hair treatments and it must not leave any residues of its own.
It must be non-toxic and reasonably non-irritant to skin and eyes. It is these
requirements that have made ammonium lauryl sulfate (ALS) and sodium laureth
sulfate (SLES) the dominant primary surfactants for so long.
Foam: [12]
Foam is also a function of the primary surfactant and few materials can compete with ALS or SLES for quick flash foam. Additional materials may depress the foam or make it creamier and stabilise it. Dialkanolamides were the firm favourite for three decades but are increasingly being replaced by amphoteric surfactants.
Conditioning:[12]
There are many ways of improving hair
conditions. SLES and other anionic surfactants leave the hair feeling dry and
difficult to manage. The introduction of a suitable secondary surfactant
greatly reduces this.
Viscosity:
[12]
Products must have sufficient
viscosity to stay on the palm of the hand prior to application but must not
come out of the bottles as a globular lump. Anionic systems may be thickened by
the addition of electrolytes or non ionic compounds or by betaines. Sodium
chloride and cocamidopropyl betaine (CAPB) are the materials of common choice.
Aesthetic appeal:
[12]
Although color, odor and pretty
pictures on the label are essential factors for aesthetic appeal, the product
appearance is also important. It must be either opaque or clear. Clarity
requires complete solubilisation of all ingredients, something that can be
surprisingly difficult despite the high level of surfactants present.
Traditionally polysorbate-20 and PEG-40 hydrogenated castor oil have been used
but several new materials have proved successful. PEG-6 caprylic/capric
glycerides in combination with PEG-60 almond glycerides, is useful for solubilising
essential oils and vitamin oils. PEG-18 Glyceryl Oleate/Cocoate is a good
solubiliser and also adds viscosity.
Safety and mildness:
[12]
These are essential attributes of a
product that may be used every day and which can come into contact with skin
and eyes. Alkyl Sulfates and alky ether sulfates are aggressive surfactants
that can irritate eyes and scalp and cause skin dryness. The effects are
usually modified by the addition of amphoteric or non-ionic materials.
Mechanism and Theory in Cleaning Process
The colloid and surface phenomena in Shampooing
Surfactants are molecules that have
the ability to be both hydrophobic and hydrophilic. This is achieved by having
two very different functional groups attached to each other. The hydrophobic
part of the molecule usually consists of a hydrocarbon of variable length.
Common chain lengths are between C8 and C18, the most used being C12 in
cosmetics formulations. This strikes the balance between mildness and
detergency or the ability to remove grease from the hair. Shorter chain lengths
have stronger grease removing properties, longer chain lengths have greater
mildness but less lathering properties and a balance has to be achieved in the
formulation.
The hydrophilic part of the molecule
can be of many and varied functional groups and will determine the nature of
the surfactant and a lot of its properties. These include sulphate, ethoxy
sulphate, succinates, polyhydroxylates, quarternerised groups and many more. [11]
Cleaning action works like this: The hydrophobic end secures itself in the oily layer of sebum
while the hydrophilic end remains anchored in water. As the hair is rinsed, the
soiled sebum is washed away.
Most shampoos are synthetic surfactants
and act by surrounding tiny oil and dirt globes in an aggregate called
micelle. These are spherical groupings
of 40 to 100 molecules in which all hydrophobic ends point toward the center
and all hydrophilic ends stick out in the surrounding water. Cleaning action takes place as the
hydrophobic end secures itself in the oily layer of sebum while the hydrophilic
end remains anchored in water. As the
hair is rinsed, the soiled sebum is washed away as the micelle. Removal of the sebum is facilitated by the
ability of surfactant molecules to form these micelles. Any tiny oil droplets removed from the hair
by the surfactant will be attracted to the center of the micelle, keeping the
oil drops from coalescing and re-depositing themselves on the hair before they
can be rinsed away.[7]
Surfactants have a polar and no polar
region. At low concentration,
surfactant is evenly distributed. At high concentration, the surfactant form
micelles. The most hydrophobic molecules will bind to hydrophobic region on the
surfactant micelle. Less hydrophobic molecules will loosely bind to the
micelle. Small molecules in the electrolyte move faster than molecules
associated with the surfactant micelle. The voltage causes the negatively
charged micelles to flow slower than the bulk flow (endoosmotic flow). This is
known as micellar electrokinetic chromatography, as show in Fig. 2 [17]
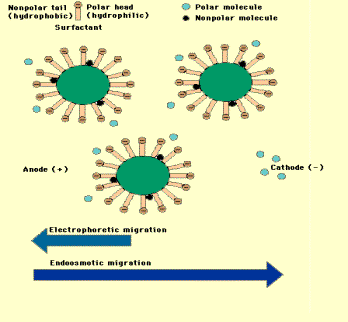
Fig2.[17]
Surfactants also contribute to
cleaning in a completely different way by affecting a physical property of
water known as surface tension. Water
molecules are electrically charged with regions of positive and negative
charge. The reason is that the two
hydrogen atoms are not symmetrically distributed around the oxygen atom but are
on the same side. As a result, the
negative area of one molecule is attracted to the positive region in an
adjacent one. This is why water forms
beads on a surface or drops in the air; the surface area of the water in
contact with the air is minimized because water molecules are attracted to each
other much more strongly than to the air.
To clean best, water needs a greater wetting ability. Surfactants accomplish this because their
molecules wedge between water molecules, reducing surface tension and allowing
water to flow easily into every nook and cranny on a surface.[7,18]
Lathering of a shampoo also is the
result of the activity of surfactants.
Foam is nothing more than dispersion of a gas, in this case air, in a
liquid. A close look at foam produced
by a shampoo reveals that it consists of air bubbles surrounded by a thin layer
of water. To achieve this effect, the
liquid's surface tension must be reduced so it no longer needs to minimize the
surface area exposed to air. The water can then stretch around the air
bubble. There is no clear link between
a surfactant's ability to clean and to produce foam. Indeed, very effective
shampoos that do not lather well can be formulated. But they do not sell well
either. Most shampoos incorporate
surfactants with strong lathering properties although they may not be ideal in
terms of conditioning or irritant potential. [7,18]
Mechanisms of Cleansing [12,18]
The mechanisms of hair cleansing are complex. Undamaged hair has a hydrophobic surface to which lipids are strongly adsorbed. When hair is shampooed anionic surfactants are adsorbed to hair by their hydrophobic tails and the negatively charged heads orientate outwards. The fiber surface is thus wetted and non-polar materials are displaced. Semi-polar materials are solubilised into micelle structures and are removed by rinsing. Particulate matter adheres to hair through ionic and van de Waals forces, which are much reduced by the surfactant system and it is readily removed by rinsing.
When comparing surfactant systems the
different mechanisms involved in cleansing should be considered; they are
mostly favored by anionic surfactants but non-ionic ones have an important part
to play in solubilisation processes. The solubilisation process depends on the
critical micelle concentration (CMC) and the number of micelles that aggregate
together. Large micelles make large aggregates, which make it easier to absorb
lipids within the micelle. Non-ionic surfactants and electrolytes pack
themselves between the micelles of anionic surfactants, which increases the
size of the aggregate and improves the solubilising of lipids. Thus ALS has a
lower CMC and a higher aggregate number than SLS and is therefore a more
effective cleanser. Ether sulfates have larger micelles and more are involved
in micelle formation so they are more effective than non-ethoxylated alkyl
sulfates.
Viscosity and Rheology properties in Shampoo [19,20]
Shampoos are rather concentrated aqueous solutions of mostly anionic surfactants in combination with salts, particularly sodium chloride. In addition to foaming, detergency and mildness to the skin, the rheology of these liquids is of key importance. In use, the consumer expects the liquids to have non Newtonian flow behavior, i.e. A slow flow from the bottle, indicating a high active content and allowing optimum dosage and easy distribution on hair. Flow behavior in which long thin threads or even cobwebs are formed when the bottle top is lifted is undesirable.
The characterization of the flow
behavior has hitherto been limited mainly to the measurement of flow curves,
i.e. recording the shear viscosity as a function of the shear rate γ . Typical viscosity profile shows a pronounced
pseudoplastic behavior. The shear rate ranges applicable to actual use are
about 5-10 s-1 for flow from the bottle and about 50 100 s-1 for distribution
on the hair.
Surfactants are compounds, which,
above a critical concentration, form micelles, i.e. aggregate which are
undergoing a continuous dissolution and reformation process. Depending on the
molecular structure, the concentration and or other additives, these micelles
have a spherical or an isometric shape, in particular, rods. In contrast to the
largely monodisperse spherical micelles, the rod shaped micelles are
polydisperse. Their average length increased with surfactant concentration.
As
long as the rod shaped aggregates are smaller than the average distance
between them, the viscosity is low, similar to that of the solvent. Surfactant
of electrolyte addition causes growth of the micelles until they overlap,
whereupon the viscosity usually increased considerably, by many orders of magnitude.
This is due to the formation of thread like, flexible micelle, which entangle
one another, separate, and hook together again, thus building up networks that
are temporary, similar to the micelles themselves. These dynamic networks, in
higher and lower magnification, are indicated in Fig. 3.
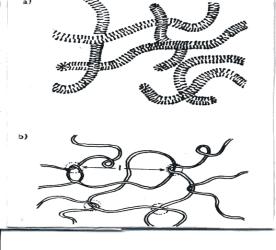
Fig 3. Temporary networks (a) Flexible rod-shaped micelles;
(b) entanglement network.[19]
The viscoelasticity typical for such
polymer net works is also shown by certain aqueous surfactant solutions,
sometimes even at low to emidilute concentrations, as is well documented for
cationic surfactants complexes with strongly binding organic counterions such
as substituted benzoic acids or in the presence of very high salt concentrations.
The rheology of many viscoelastic surfactant solutions follows the behavior of
Maxwell fluid. This means that its rheology can be described the simplest
mechanical model. It consists of just one dashpot (for the lost energy
equivalent to the viscosity) and one spring. (For the stored energy, equivalent
to the elasticity).
Instead of forming thread like
micelles, which build up a transient entanglement by shielding the micellar
charge of the anionic surfactants, shampoos are also formulated with polymers
in order to achieve suitable rheological properties. Viscosity is simply
achieved through the addition of electrolyte, usually common salt. Salt
thickens shampoo due to the ability of the sodium ions to lower the charge
density on the outside of the micelles in the shampoo. This usually only
applies to anionic species or anionic nonionic mixtures.
The viscosity of a surfactant mixture is dependant upon the
size of the micelles in the system. This is determined by several factors -
concentration of surfactants, the type and ratio of species, temperature and
charge density on the micelles. In mixed micelles the arrangement of the
molecules is limited to some extent by the charge density on the surface of the
micelle. This can be reduced in several ways. One is addition of more nonionic
surfactant so that the ratio in the micelles rises and the negatively charged
heads of the anionic surfactants are held further apart. Another is addition of
a substance that reduces the charge density on the surface of the micelles.ie
salt. By doing this charge density drops and the micelle size can increase. It
also causes a rise in micelle aggregation number and the transition from
spherical to cylindrical micelles. This transition also leads to a jump in the
formulation viscosity as the threads can get knotted around each other. If this
process continues a lamellar structure can be formed which is a gel. However it
is very variable the response to salt addition in the system. If too much salt
is added to a mixture then it can "crash". This is a sudden
transition from thick back to thin liquid. In some cases this can be reversible
with the addition of water. But too much salt reduces the solubility of the
surfactant so much (again the equilibrium is forced to the left) that it comes
out of solution and precipitates.
Water soluble polymers bear a
sufficient number of hydrophilic groups that interact strongly with the water
structure. They form solvation shells, which open the coil structure. Instead
of conventional or high molecular weight polymers, cosmetic chemists often use
thickeners that are efficient specifically in aqueous surfactant solutions such
as PEG- (75-150) distearate or dioleate, PEG 50 Ppo 1 dioleate etc. those
compounds are rather low molecular weight polymers, which display very low
viscosity on their own, are likely non ionic surfactants. As the mode of
action, mysterious synergistic interactions between surfactant micelles and
polymers happen. Synergism leads to nothing else but the formation of long
thread like micelles because of charge shielding.
Test methods for surfactants in shampoo
Ordinary methods[21]
Generally, there are two types methods
for surfactants analysis in shampoo, one is qualitative examination and the
other is quantitative analysis.
Qualitative examination:
1. Behavior on acidification: the first step in the qualitative examination is to acidify an aqueous solution of the product with hydrochloric acid. If the later persists the presence of a surfactant is indicated.
2.
Test for elements: if
a surfactants is present, a filtered ethanol extract of the product is tested
for sulphur, nitrogen, phosphorus and halogen either by the sodium fusion
method or preferably by the zinc dust sodium carbonate fusion method;
3.
Test with mixed
Dimidium Bromide Disulphine Blue Dyes: in the presence of an anionic
surfactant this indicator imparts a pink color to the chloroform phase. In the
presence of cationic matter the chloroform phase is colored blue.
4.
Test with methylene
blue solution: depends on the chloroform phase color can determine cationic,
anionic or nonionic surfactant may be present;
5.
Test with Pontamine
Fast Red 8NL: reactions with this dye
are carried out in an alkaline solution. Under these conditions the surfactant
is anionic if the red color is initially in the aqueous phase. If the color is
initially in the chloroform phase, the surfactant is cationic;
6.
Paper chromatography:
it permits the identification of a number of different types of active matter,
of organic bases and of a number of additives which may be present;
7.
Heating with
Phosphoric Acid: when dry oxyethylene or oxypropylene adducts was heated with
phosphoric acid, the oxyalkylene chain was degraded to give acetaldehyde or
propionaldehyde respectively.
Also Thin layer chromatography,
Effect of Hydrolysis and Heat, Preliminary Deductions are qualitative
examination methods.
Quantative analysis of anionic surfactants
Depending on the different function
groups of anionic surfactants, there are different quantative methods; one of
general methods is volumetric titration. Titration techniques were among the
earliest methods developed for the analysis of anionic surfactants. They
included:
-
Acid base titration:
-
Single and two phase
titration with cationic active matter
-
Double
decomposition reactions;
Quantative analysis of cationic surfactants
Generally, there are
small amounts cationic surfactants in shampoo; about five methods can be used
to test them.
-
Gravimetric method;
-
Volumetric method;
-
Colorimetric method;
-
Chromatographic
techniques;
-
Ultraviolet
spectroscopic method
New methods:
1. Flow injection
analysis:[22]
The flow injection analysis (F I A)
method based on the extraction of the CS with anionic dyes, i.e. Orange II,
tetrabromophenolphthalien ethyl ester etc was not good enough. The selectivity
and sensitivity of these methods are very poor with low sample throughput and
precision. A new, simple and specific FIA method for the determination of CS in
the term of cetylpyridinium chloride (CPC) based on the enhancement of color
intensity of the Fe (III) SCN complex in the nitric acid medium was
developed.
2. Prediction of
ocular irritancy [23]
- Isolated rabbit eye (IRE)
The isolated rabbit test was first
proposed by Burton et al (1981), as a means of screening for severe irritants
without using live animals. The IRE confers the advantage that animals are not
bred exclusively for the purpose. The method is capable of distinguishing
between mild and moderate eye irritants, such as baby and normal adult
shampoos.
- Bovine corneal
opacity and permeability (BCOP) assay
The BCOP assay was introduced by
Gautheron et al (1992) as a method for screening process intermediates for
worker safety. BCOP data correctly predicted whether a compound would be
irritating or non irritating.
Both methods use the metric of corneal
opacity, which in relation to accidental human exposure to an irritant is
arguably the most important parameter, since it provides information on how
visual acuity may be impaired accidental exposure.
Shampoo Production
General process
Shampoo in terms of product consists
of surfactants (cleaning agents) and conditioners. Surfactants, which are
mainly detergents like AOS - alfa olefin sulphonate and LES (lauryl ether
sulphate salt) are suspended in distilled water with perfume. Conditioning
agents are added which could be different silicones or cationic polymers. For
general production, fist dispersing thickener in deionized water, then add some
PH adjuster, then add surfactants, preservatives and other additives, at last
final PH was adjusted to 5.0 6.0. After the system cool down, add fragrance.
In all the process, continuing stir is needed in order to guarantee all
chemicals and water mix completely. Shampoo is then filled in bottles or
sachets. Packaging is technology intensive.
BRAN + LUEBBE System [24]
BRAN + LuEBBEs shampoo blending
system is a compact, closed, multistream continuous proportioning system with
variable delivery, positive displacement pump for each stream and a common
drive for all the pumps. Drive speed can be fixed or variable to suit the
application. The system was showed in Fig5.
Fig5 BRAN+LUEBBE system
Components:
Multi gang Bran
+Luebbe AREX metering pump
In line mixing
PH and viscosity
monitoring system
Process
The
typical system for the production of shampoos, supplied completed with
instrumentation and pipe work, produces many variants. The process starts with
the surfactants and the water being metered and passing through the first in
line mixer. The brine and one of the four additives to produce the required
recipe are added just prior to the second in line mixer. Immediately after
this stage, the mixture passes through a small vessel, which contains the
viscosity measurement.
The PH measurement is carried out after
this vessel. The finished product now goes to a buffer tank in which a level
control automatically adjusts the total output of the blending unit according
to the demand of the filling machine. Additional items are the glass suction
vessels for each constituent. These are fitted with low-level alarm probes for
loss of liquid. A three way valve, fitted up stream, enables, flushing water to
be pumped through the system for rapid in place cleaning and product change
as necessary.
Key advantages
The major advantages of the BRAN + LUEBBE
proportioning systems are:
-
Substantial savings
in production space, manpower and costs;
-
Product uniformity
and total quality control;
-
Fast, easy recipe
changing without wastage;
-
The option to
automate any or all production functions;
-
Closed system
produces a sanitary, air free product.
Marketing
consideration
Shampoo is a high margin product and contribution margin is around 50-60% of realizations. Out of total direct cost, raw materials account for 40-45% and packaging materials account for 25-30%. The balance is accounted for utilities etc. Advertisement costs are substantial at about 15% for established brands. A nation wide launch costs about Rs100-150mn.[25]
The direction of the hair care market, whether it is moisturizing or coloring, is heading towards making life easier. Another trend in the market is form more natural products.
In 1997 in American, for years drug
store and supermarket shelves have been filled with $1.99 shampoos and
conditioners. To get the more expensive stuff, consumers had to strike out for
the nearest salon. To meet consumer demand, many companies developed new,
luxury products[26].
In 1998, with the economy still booming and Americans feeling comfortable disposing of their disposable income, pampering themselves has become commonplace, and hair care is one of categories benefiting from the high-flying economy. According to IRI, shampoo sales rose 7.6% to $1.6 billion. Unit sales were up only 2.8%, however, to 630 million. By brand, Pantene held the top spot with a dollar share of 14.7% and was second in unit share with 11.5%. Herbal Essence (9.1%) head and Shoulders (8.4%), Suave (7.6%) and Pert Plus (6.6%) rounded out the top five in dollar share.[27]
In 1999, a combination of innovative
technologies from research and development departments and the consumers
desire for healthy hair has led to a boom in the hair care market. P&G
power brand, led the shampoo category with annual sales of $237.3 million,
respectively for the year ended July 18, 1999 by IRI.[28]
In December 2000, markets kept $1.76
billion shampoo sale, according to IRI. Pantene, the mass-market sales leader
in shampoo with $238 million, has ventured out in new bottle collections to
give customers selections based on desired end look.[29]
Antioxidant, Vitamin enriched and
natural is the terms to which more and more consumers are responding when
determining which hair care products to buy in 2001. According to information
Resources Inc., shampoo sales for the year ended Aug. 12 rose 1.2% to $1.79
billion in supermarket, drug store and mass merchandisers.[30]
Conclusions
- Hair chemistry structure is the
foundation of shampoo design
- Shampoo generally is composed by
cleaning agents, modifying agents, PH adjuster and Fragrance oil etc. For
all of the ingredients in shampoo, the most important are surfactants;
- The major types of surfactants are
anionic, cationic, nonionic and amphoteric. Anionic surfactants are
primary detergents and cationic surfactants have good condition
properties. Nonionic surfactants aid in solubility, modifying foam.
Amphoteric surfactants are gentle and wont burn the eyes, are best choice
for baby shampoo.
- Surfactants will influence six
essential attributes of shampoo: cleaning, foam, condition, viscosity and
aesthetic appeal combined with safety and mildness in use.
- Surfactants are molecules that
have the ability to be both hydrophobic and hydrophilic. They affect the
surface tension of water. The cleaning mechanism is the hydrophobic end
secures itself in the oily layer of sebum while the hydrophilic end remains
anchored in water. As the hair is rinsed, the soiled sebum is washed away.
- Above a critical concentration,
surfactants form micelles. The viscosity of shampoo depends upon the size
and shape of micelles. This is determined by several factors - concentration
of surfactants, the type and ratio of species, temperature and charge
density on the micelles. In mixed micelles the arrangement of the
molecules is limited to some extent by the charge density on the surface
of the micelle. This can be reduced in several ways. One is addition of
more nonionic surfactant, another is adding electrolyte.
Reference:
1. http://www.geocities.com/HotSprings/4266/chem.html;
2. http://hometown.aol.com/hairbook/know.htm;
3. http://www.hairless.net/hairbiol.html;
4. http://www.surfactants.net/formulary/uniqema/pcm6.html;
5. http://www.fmhs.uaeu.ac.ae/females/shampoo.htm;
6. http://www.satisfied-mind.com/drugstore/shampoo.htm;
7. http://www.washingtonpost.com/wp-srv/national/horizon/dec98/shampoo.htm;
8. http://www.halifax.cbc.ca/streetcents/show/more/show_08_00/shampoo.html;
9. http://www.hair-shampoo.com/;
10. http://crystal.biol.csufresno.edu:8080/projects97/115.html;
11. http://www.hairscientists.org/article7.htm;
12. http://www.creative-developments.co.uk/
13. Ronni Wolf. MD, Clinics in
Dermatology, 2001; 19: 393-397;
14. Kirk-Othmer Encyclopedia of
Chemical Technology, 4th ed., volumes 1-24;
15. Estrin, Norman F., Crosley,
Patricia A., Haynes and Charles R., CTFA Cosmetic Ingredient Dictionary, 3rd
ed., Washington, D.C., Cosmetic, Toiletry, and Fragrance Association, c1982;
16. Robbins, Clarence R., Chemical and
Physical Behavior of Human Hair, 3rd ed., New York: Springer-Verlag,
c1994;
17. http://ntri.tamuk.edu/ce/surfactant.html;
18. Martin M. Rieger, Surfactants in
Cosmetics, New York and Basel, 1985;
19. D.Balzer and M. Weihrauch, Colloids
and Surfaces A: Physicochem. Eng. Aspects 99(1995) 233-246;
20. http://www.hairscientists.org/article15.htm;
21. G.F. Longman, The Analysis of
Detergents and Detergent Products, 1975.
22. Rajmani Patel etc. Talanta, 48(1999) 923-931;
23. K.J. Cooper etc, Toxicology in
Vito, 15(2001)95-103;
24. http://www.bran-luebbe.co.uk/shampoo.htm
25.
http://www.indiainfoline.com/sect/pchc/ch06.html;
26. http://www.happi.com/special/GENERAL/aug972.htm;
27. http://www.happi.com/special/1208mm1.htm;
28. http://www.happi.com/special/dec993.htm;
29. http://www.happi.com/special/dec001.htm;
30.
http://www.happi.com/specials/Deco11.htm;