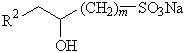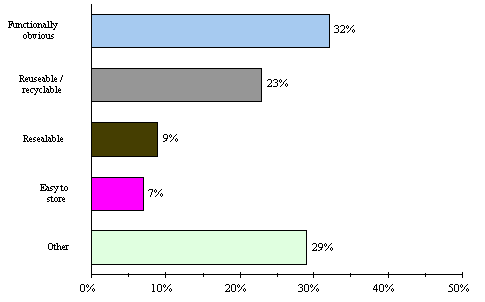Colloidal and Surface Phenomena of
Liquid Laundry Detergent
CE 457-527
Dr. Alexandridis
April 9, 2002
Daniel Boek
Erika Indivino
Katherine Marso
Karey Smollar
Table of Contents
Page
History 3
Components 8
Soil Removal 16
Fabric Types 27
Processing 28
Packaging 28
Environmental Concerns 30
Market Sales 30
History and Background of Laundry Detergents
The method of cleaning clothes has changed greatly since its beginnings in ancient history. The first form of laundering was purely mechanical. Clothes were beaten against rocks to remove water-soluble stains. To remove the more difficult oily stains, additional compounds needed to be added. This led to the first production of soap in the 15th century[1].
By combining animal or vegetable fats with aqueous sodium hydroxide soap is made. Soaps are advantageous because they are made from biodegradable renewable resources. Therefore, soaps are not polluting the environment. These factors are outweighed by the negative affects of hard water and the cost of the raw materials. Soaps react with calcium and magnesium ions in hard water leading to the formation of precipitates. Increased household use of alkylbenzene sulfonates (ABS), a former compound used in laundering with soaps, resulted in large bodies of water covered in foam. The synthesis of ABS is shown below in Figure 1.
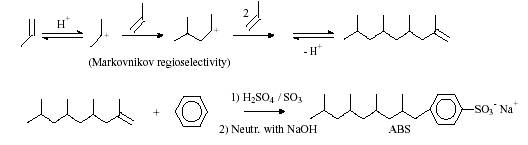
Figure1: Synthesis of ABS[2]
These factors, as well as the commercialization of the Ziegler process leading to the production of linear alkylbenzene solfonates, lead to the production of synthetic detergents[3].
The manufacture of synthetic detergents, commonly called laundry detergent, began in 1916 in Germany during WWI. The production of detergents in the United States took off after WWII. The first detergents were used mainly for dishwashing and fine fabric laundering. In 1946 the first all-purpose laundry detergent was produced using builders with surfactants[4]. Surfactants and builders became increasingly more complex as the demand for better soil removal grew. The use of sodium triphosphate (STP), a very effective builder, was restricted in the 1960’s because it caused eutrophication in rivers. Trisodium citrate, NaCit, is an effective, biologically degradable builder commonly used in detergents today[5].
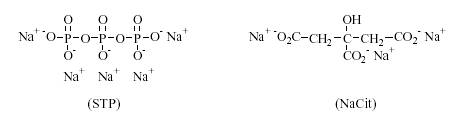
Figure 2: Chemical Structures of STP and NaCit[6]
New additives are continually being introduced to the detergent industry to keep up with customer demand.
Liquid laundry detergent became popular in the U.S. in the 1970’s. The USA holds the largest market of liquid heavy-duty detergents (HDL) in the world. Liquid laundry detergents usually do not contain bleaching agents and are best at removing oily stains at low temperatures[7]. Liquid laundry detergent is a huge industry in the United Stated. Research has continued to develop detergents that would provide people with efficient cleaning agents that are safe for the environment. The sale of liquid detergent has soared over powdered detergent in the last decade. The two types have reached a 50/50 market split in the U.S., whereas liquid detergent only holds 13% of the market in Europe. Consumers find liquid detergents more convenient to use while giving better results in the U.S. Liquid detergents are easier to measure and easier to apply directly to stains. The liquid laundry detergent industry will continue to grow in the coming years and overtake that of powdered laundry detergents[8].
An interesting development in the industry is the introduction of tablets first marketed in 2000. Tablets are marketed towards students and the elderly for their convenience factor. Tablets dissolve within seconds of hitting the water. Although they are new and fairly expensive, with continual improvements in efficiency and production, tablets will also become a strong competitor in the market[9].
There has been very little response to tablets in the U.S. In Europe, on the other hand, tablets hold 25% of the market in some countries. This response lead to the introduction of the sachet, or pouch, in April 2001. This new product delivers liquid laundry detergent through a water-soluble polyvinyl alcohol skin. The pouch dissolves in minutes, leaving behind no residue. These types of unit-dose products, such as tablets and pouches, are on the very frontier of the detergent industry. It is a race to see which company can produce the fastest dissolving, most easily dispensed detergent on the market[10].
Design considerations
Laundry detergent is a basic necessity for every household. Liquid laundry detergents must have specific properties that will meet the needs of the general public.
Excellent soil removal is the main feature that
consumers look for when choosing a detergent.
This is the central reason that people need any cleaning agent, to
remove the dirt.
Low sensitivity to water hardness is another crucial
property of detergents. Many homes only
have hard water available. Hard water
that contains high concentrations of calcium and magnesium greatly decreases
the effectiveness of soaps. The
minerals react with the soap to form a precipitates that are left on
clothes. Detergents were introduced to
laundering applications to remove soils without leaving precipitates. Since most people cannot afford to have a
water softening system their home, it is important that detergents avoid the
formation of precipitates. Liquid
laundry detergents contain builders that prevent calcium and magnesium deposits
when using hard water. Detergents must
also have good dispersion properties to ensure that the entire load of clothing
is sufficiently cleaned. Liquid
detergents dissolve and spread much faster than powder detergents do in water. Soil antiredeposition capability is also
very important. Surfactants in the
detergent must be decided upon according to how well they keep soil from
redepositing onto the clothing. The
soil from the clothing must be kept in suspension in the water until it can be
rinsed away. It is important that
detergent has a high solubility in water.
The purpose of using a detergent is to overcome the surface tension of
water so that soils can be removed. The
surfactants need to overcome the surface tension of water to ensure sufficient
wetting power. Liquid laundry
detergents dissolve in water more quickly than powdered laundry detergents,
especially in cold water.
The amount of foam during washing has a psychological
affect on whether detergent is or isn’t working. Too little foam indicates poor cleaning performance. Poor rinsing and draining is also a result
of too much foam. Liquid laundry
detergents contain surfactants that act as foaming agents to control this
element of the detergent. Odor is
another aspect to be considered when thinking about consumer needs. Fragrances need to be added to cover any
chemical smell of the detergent.
Perfumes are also added to differentiate one brand from another. Detergents with low levels of fragrances are
also produced for people who are sensitive to perfumes. The color of the product should also be
considered, but not too excessively. On
the other hand, the amount of toxicity to humans should be a major
concern. One cannot assume that every
user of a household product pays strict attention to warning labels or recommended
safety precautions. Therefore, exposure
through skin, ingestion, and inhalation must be investigated whenever chemical
products are put on the market. Every
compound included in liquid laundry detergents is extensively investigated for
its affects on humans.
Favorable environmental behavior is another important
aspect that must be integrated into liquid laundry detergent. Excess water containing additional energy
(heat), soil from the laundry, lint, dyes, finishing agents, and detergents is
drained into the environment[11]. The affects of these by-products on the
environment need to be as minimal as possible.
The use of phosphates, one of the original compounds used in detergent,
has been severely limited due to their harmful affects on the environment. Companies continue research in the area of
environmental affects to ensure that detergents are not damaging the
environment.
Convenience is a
very important design consideration for detergent companies. Costumers find liquid detergents easier to
pour with a cap as opposed to scooping dry detergent out of a box[12]. All the above mentions aspects of detergent
must be integrated into product that can be sold in a very competitive
market. There is a fine balance between
creating an efficient cleaning agent, keeping the costumers happy, and keeping
the environment safe.
Components
The components of liquid laundry detergent are listed
in Table 1[13] below:
Table 1:
Composition of liquid laundry detergent
Ingredients
|
Volume Percent
|
|
Anionic
Surfactants |
10 - 25 |
|
Nonionic
Surfactants |
6 - 10 |
|
Soaps |
4 - 6 |
|
Builders |
15 - 30 |
|
Solubilizers |
0 - 5 |
|
Alcohols |
0 - 5 |
|
Enzymes |
0 - 1.5 |
|
Optical
Brighteners |
0.05 -
0.25 |
|
Stabilizers |
Trace
amount |
|
Fragrances |
Trace
amount |
|
Water |
30-50 |
Surfactants
Surfactants are the most important components of
liquid laundry detergents. Surfactants
are water-soluble surface-active agents that adsorb onto the surface of soil
particles to separate the soil from clothing.
Surfactants remove oil by lowering the surface tension of water,
allowing the clothing surface to become wet.[1] Surfactants consist of a hydrophilic and a hydrophobic
portion. The hydrophobic component
consists of an eight to eighteen carbon hydrocarbon. Sources of these hydrocarbons are natural fats and oils, petroleum fractions, synthetic
polymers, and synthetic alcohols.
Surfactants are divided into three groups; anionic, nonionic and cationic. Anioinic surfactants are the most abundant ingredient in liquid laundry detergent because they have proven to show the most enhancing effects of removal of soil. Nonionic surfactants are used primarily for their stabilization effects during detergency.
Surfactants are chosen based on their sensitivity to water hardness. Certain surfactants such as Linear Alkylsulfonate (LAS) show good detergency despite water hardness. The surfactants exhibiting more sensitivity to water hardness are less effective regarding their absorbance to fabrics and an increased production of surface film. Not one single surfactant is capable of effectively removing all soil types on different types of fabrics. Therefore, surfactant mixtures are proven to be most effective when considering a wide range of solvent conditions and soil types.
The effectiveness of surfactants is proportional to
the length of the chain. A high number
of carbon atoms in the surfactant molecule correspond to an increase in the
number of surfactants adsorbed. The
type of hydrophilic group determines the classification of the surfactant as
anionic, nonionic, or cationic. The
characteristics of surfactants cause the hydrophobic component to be drawn
together to form micelles. This allows
the surfactants to form a coating around a suspended material. A surfactant used to suspend a solid in
water is called a dispersant.[2]
Liquid laundry detergents contain larger amounts of anionic
surfactants than ionic surfactants.
Anionic surfactants ionize in solution and have a negative charge. They are good cleaning agents and create
high “sudsing”. Some examples of
anionic surfactants are linear alkylbenzene sulfate, alcohol ethoxysulfates,
alkyl sulfates and soaps. Nonionic
surfactants do not ionize in solution and therefore have no electrical charge. Cationic surfactants ionize in solution to
give a positive charge.
Anionic Surfactants
Anionic surfactants are the main component of liquid laundry detergents and found to be the most active ingredient for the soil removal process[14]. Some examples of the most common type of anionic surfactants include sodium linear alkylsulfonate (LAS), alkanesulfonates (SAS), and olenfinsulfonates (AOS).
The production of LAS originated from the former surfactant called Tertrsproplynenbenzene sulfonate (TPS), which was used in the earlier stages of detergency to replace the use of regular soaps. The structures of both molecules are similar, but the LAS molecule eliminated the branching found on the TPS molecule. The straight chain structure of LAS demonstrates more effective detergency due to its increasing solubility, greater level of biodegradation, and less sensitivity to changing pH levels. LAS is shown below in Figure 3.
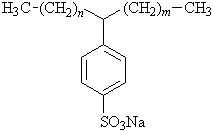
n + m = 7–10
Figure 3: Sodium linear alkylsulfonate (LAS)
TPS is shown below in Figure 4.
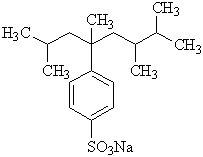
Figure 4: Tetrapropylenebenzenesulfonate (TPS)
Another type of anionic surfactant, Sodium Alkanesulfonate, has high solubility, fast wetting properties, and chemical stability of alkali and acids[15].
The production of SAS is formed by sulfooxidation and sulfochloronation process.
Figure 5: Secondary Alkanesulfonates (SAS)
The third type of anionic surfactant, Olenfinsulfate (OAS) is produced using the alkaline hydrolysis process. This surfactant is unique because it exhibits less sensitivity to water hardness. This effect can be dependent on the chain length of the hydrophobic portion of the surfactant.
|
R1–CH2–CH=CH–(CH2)n–SO3Na |
Alkenesulfonates |
|
|
|
|
|
Hydroxyalkanesulfonates |
|
|
|
|
R1 = C8 – C12 |
n = 1, 2, 3 |
|
|
|
|
R2 = C7 – C13 |
m = 1, 2, 3 |
|
|
|
Figure 6: Olefinsulfonates
(AOS)
The characteristics of these surfactants offer several advantages over one another. Due to the fact that detergents must maintain satisfactory ability for removing various types soils, the most effective type of surfactant used has proven to be a surfactant mixture. The advantages of using surfactant mixtures include reduction of amount of detergent needed during the detergency process and the reduced size of packaging.
Nonionic Surfactants
Nonionic surfactants are also an essential ingredient for liquid laundry detergents, but are often found in a much smaller quantity as compared to anionic surfactants.
Both types of surfactants play different roles in detergency but work well in conjunction with one another. The anionic surfactant plays an active role in the removal of the soil from the surface of the fabric. The nonionic surfactants are used for the stability of solution. The nonionic surfactants, depending on their structure, are commonly attracted to the outer most part of the micelle and are used to help stabilize the micelle formations and reduce redeposition of the treated soil back onto the fabric.
Builders
Builders play an important role in the effectiveness of liquid laundry detergents by enhancing the affects of surfactants. Builders help remove water hardness and keep soils from redepositing onto the clothing. The most common types of builders are phosphates, silicates, carbonates and oxygen releasing materials. Phosphates are no longer used due to their negative affect on the environment.
Builders are also used to remove Ca2+ and Mg2+ ions which produce hardness of the wash liquor. Calcium and magnesium form complexes with builders, diminishing the surfactant interaction with calcium and magnesium. Trisodium citrate is the most common builder used in today’s laundry detergent. Trisodium citrate is shown in Figure 7.
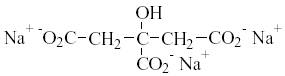
Figure 7: Trisodium Citrate (NaCit)
In the absence of builders, hardness ions form complexes with soil. The complex can redeposit onto the negatively charged fabric and soil interfaces. Zeolites are water-insoluble builders of 10mm diameter and molecular formula Na2OAl2O3*4.5H2O. Calcium and magnesium ions replace sodium ions from the zeolite crystals. Therefore, hardness ions cannot form complexes in the wash liquor.
Redeposition Inhibitors
Micelles are negatively charged structures that can redeposit on neutral surfaces. For example, synthetic fibers don’t acquire a strong negative charge in water. Therefore, the surface needs an electrostatic charge to keep negatively charged detergent micelles from redepositing their soils on the fabric. These additives are known as redeposition inhibitors. Sodium carboxymethyl cellulose (SCMC) is a polymer of molecular weight from 20,000 to 500,000 that attaches itself to the fibers and adds to the negative charge. SCMC can be seen in Figure 8 below.
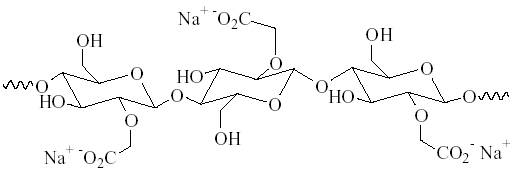
Figure 8: SCMC Diagram
The types of detergent builders
have changed over the years with increasing environmental awareness. With
tighter environmental restrictions, the uses of phosphates biulders in
detergents have been diminishing.
Enzymes
Protein stains from sources such as milk, cocoa, blood, egg yolk, and grass are resistant to removal from fibers by enzyme-free detergents, particularly after stains are dried-on. Chocolate, starch-based food stains, and greasy/fatty stains are particularly difficult to remove in low-temperature washing. Proteolytic, amylolytic, and lipolytic enzymes are usually capable of eliminating such soil during washing.
Commercial production of detergent enzymes experienced rapid expansion in recent years. For example, by 1969 nearly 80 % of the detergents marketed in the Federal Republic of Germany contained proteases as enzyme additives to detergents. Today's detergent enzymes are perfectly safe. As a result, nearly all detergents produced in Europe, North America, Japan and many other countries worldwide contain enzymes today. The effectiveness of proteolytic, amylolytic, and lipolytic detergent enzymes is based on enzymatic hydrolysis of peptide, glucosidic, or ester linkages, respectively.
Whitening agents
Fluorescent whitening
agents (FWA), or optical brighteners, are added to liquid laundry detergents to
give laundry a much whiter appearance.
FWA’s are organic compounds that convert invisible ultraviolet light
into longer wavelength visible blue light.
Optical brighteners decrease the absorption of the blue radiation that
gives clothes a yellowish tint. Due to
the fact that FWA’s also exhibit reflectance in the visible region, a build up
on the fabric may also leave marks.
FWA’s are applied through a dyeing process. Distyrylbiphenyl, stilbene, coumarin, and bis(benzoxazole) are
the most common brighteners. FWA’s are
evaluated on their stability (resistance to chemical change} and fastness
(chemical change after adsorption).
Soil Removal
Removal
of Oily/Greasy Soil by Detergents
The removal of
soil from clothing is primarily due to the wetting properties and interfacial
tensions between the washing liquid and the deposited soil. During the washing process, the most
significant interactions occur between the liquid (water) and the
surfactant. Over the time clothes are
worn, oily/greasy soils are deposited and spread evenly on the clothing. The ability of water to be in contact with
oil is referred to as the measure of wetting.
The measure of wetting is useful when describing the contact angles,
which are responsible for the removal of the soil from the fabric.
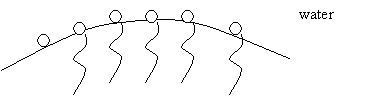
The physical properties of water and the ingredients of the detergent are essential. The soil that is present on clothing is apolar and the washing liquid is polar. In order for the removal of the soil from the clothing to occur, there must be some sort of medium, which causes a strong attraction between the two surfaces. Anionic surfactants allow for the formation of the droplets, but nonionic surfactants create the attraction between the micelles.
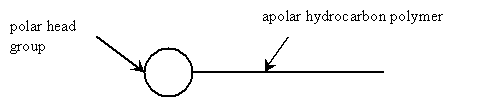
Surfactants concentrate at water-liquid, water-gas, or water-solid interfaces because the hydrophobic and hydrophilic properties of the surfactant are satisfied. One nonionic surfactant molecule has an apolar hydrocarbon polymer tail and a polar head group. The following diagram depicts a single surfactant. Another type of surfactant, ionic surfactants, have static charges associated with their head groups.
Figure 9: Nonionic Surfactant Molecule
Oil
Figure10 below displays the alignment of surfactant molecules
at an interface between oil and water.
Water
Figure 10: Oil-Water Interface
interface
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]() Before surfactants are added to the oil-water
system, the oil-water interactions are very weak. As the surfactant molecules replace water at the oil-water
interface, the attraction between water and the head group increases. Likewise, the attraction between the polymer
chain and oil also increase. The new
attractions decrease the soil-water interfacial surface tension.
Before surfactants are added to the oil-water
system, the oil-water interactions are very weak. As the surfactant molecules replace water at the oil-water
interface, the attraction between water and the head group increases. Likewise, the attraction between the polymer
chain and oil also increase. The new
attractions decrease the soil-water interfacial surface tension.
The water, soil,
and fabric interfaces are shown below in Figure 11.
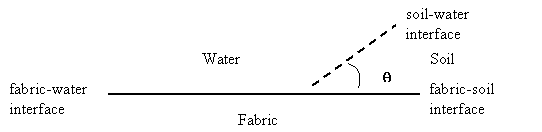
Figure 11: Contact Angle in
Water-Soil-Fabric System
The Young equation
describes the overall shape of a droplet of oil at the three-component
interface. The Young equation is
![]() (1)
(1)
where g is the surface tension at the corresponding
interfaces and q is the contact angle in Figure 11 above. After surfactants are added to the system the fabric-water and
soil-water interfacial tensions approach zero (gFW and gSW = 0). The interfacial tension
between soil and fabric remains constant, therefore, gFS > gFW. The Young equation now gives
cosq < 0.
Solving for the contact angle, q > 900. This contact angle means that the area of contact between soil
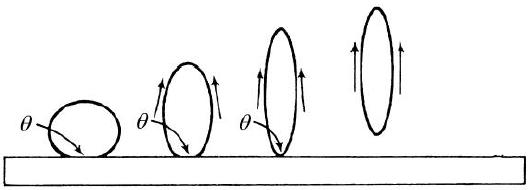
and fabric reduces to zero. Figure 12[16]
shows this mechanism of detergency, called roll-up.
![]()
Figure 12: Complete Removal of
Oil Droplets by Roll-up
Without
surfactants, part of the soil droplet can be removed from the fabric by
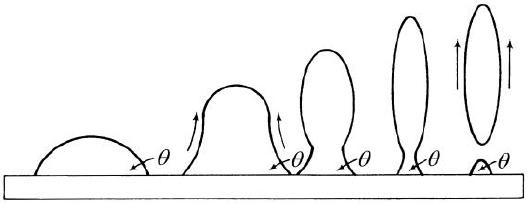
mechanical agitation. The Young
equation now yields a positive value for cos q, resulting in a positive contact angle less
than 900. As the area of
contact is reduced, the oil droplet pinches off and leaves some oil
residue. This process is illustrated in
Figure 13[17].
Figure 13: Partial Removal of
Oil Droplets by Agitation Without Surfactant
At high concentrations, detergents form micelles in aqueous solution. Micelles dissolve the fatty stains. The inner section of the micelle contains hydrophobic polymer chains and oil. Micellar size and shape can vary, depending on surfactant type, temperature, and the presence of salts. The packing properties of surfactants are dependent upon cross-sectional surface area of the headgroups (a0 ), the volume (v ) of the hydrocarbon chains, and the maximum length, (lc ), of the chains. The packing parameter is dimensionless where p=v/aolc. The packing parameter determines the geometry of the surfactants. The possible shapes are shown in Figure 14[18].
Figure 14: Geometry of Surfactants
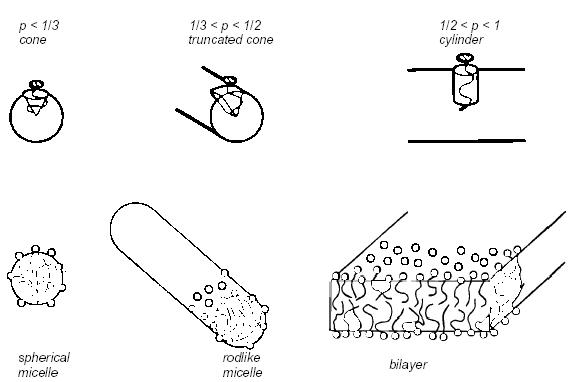
As seen above in Figure 14, for p < 1/3, the surfactant forms a cone shape. Therefore, the corresponding micelle is spherical. The truncated cone surfactant shape is valid for
1/3 < p < ½, forming a rod-like micelle. If ½< p <1, the surfactant is cylindrical in shape and a bilayer is formed.
Spherical micelles in the presence of an aqueous salt solution form a multilamellar structure. The multilamellar structure is formed because the salt ions are attracted to the surfactant headgroups. The repulsive forces between headgroups are minimized. Figure 15 below shows the difference of headgroup area with and without salt ions present
![]()

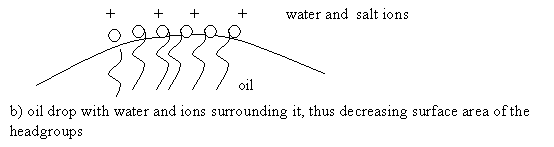
Figure 15: a) Oil with Water, b) Oil with Water and Salt Ions
The packing parameter now takes on the bilayer shape. A continuous lamellar phase of bilayer aggregates is formed (shown in Figure 16[19] below).
Figure 16: Continuous Lamellar
Liquid Crystalline Phase
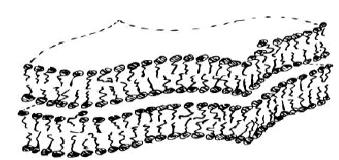
Several bilayers pack around each other to form multalamellar vesicles, or lamellar droplets. Lamellar droplets form from the continuous lamellar liquid crystalline phase even though they are metastable. Mechanical agitation dissolves sodium citrate and induces the formation of lamellar droplets. Although the continuous lamellar liquid crystalline phase is more thermodynamically stable, spontaneous curvatures of the bilayers are favored in the presence of a surfactant mixture. Bilayers form multilamellar vesicles to minimize interactions between hydrocarbon chains and solvent that would be present at the edges of the crystalline structure.
The presence of bilayers within a salt solution gives rise to the formation of multilameller (multi-bilayer) structures due to strong osmotic forces. Multibilayers are thermodynamically favored within a salt solution (i.e. sodium citrate) when the area of the hydrophilic head groups are reduced. Although the multilamellar bilayers are more thermodynamically stable, spontaneous curvature of these bilayers is favored in the presence of a surfactant mixture. This spontaneous curvature gives rise to the formation of the multi-lamellar droplets. The Gibbs energy of a two-component bilayer is minimized by the formation of the lamellar droplet. Figure 17[20] shows a lamellar droplet.
Figure 17: Lamellar Droplets
Many multilamellar vesicles phase
separate. In stable conditions
interlamellar repulsive forces are present.
Flocculation occurs if interlamellar attractive forces are strong. Van der Waals forces are present, causing
lamellar droplet flocculation.
Counterion concentration increases in the intralamellar region. Osmotic flow of solvent into the
intralamellar region takes place to keep the bilayers separated. Poor solvents increase flocculation unless
decoupling polymer is present, thus causing steric repulsion.
Flocculation of lamellar droplets causes phase separation. In the presence of electrolytes (salts) water is a poor solvent. The hydrophilic portion of the nonionic surfactant decreases in length because of this poor solvency. Therefore, intralamellar droplet volume decreases. Decoupling polymer, comprised of a hydrophilic backbone and hydrophobic side chains, is added to the solution and attaches to the lamellar droplet surface. The side chains attract to the oily portions of the exterior bilayers (of the droplet) and the backbones dissolve in the water solvent. Figure 18[21] displays three scenarios: good solvent, bad solvent, and bad solvent with decoupling polymer. Steric repulsions between the decoupling polymers causes the droplets to repel, and no flocculation occurs.
Figure 14: a) Good Solvent, b)
Poor Solvent, c) Poor Solvent with Decoupling Polymer
Figure 18: a) good solvent, b) poor solvent, c) poor solvent with decoupling polymer
Particulate Soil
The removal of particulate soil is based on the DLVO Theory. The potential energy must be overcome in order to removal the soil from the clothing fiber. Potential Energy graphs demonstrate the potential energy as a function of distance from the garment.
Calculated potential energy of attraction PA
and repulsion PR as a function of the distance of a
particle from a fabric, along with the resultant potential P;
predictions based on the DLVO theory DLVO computational parameter z = 4
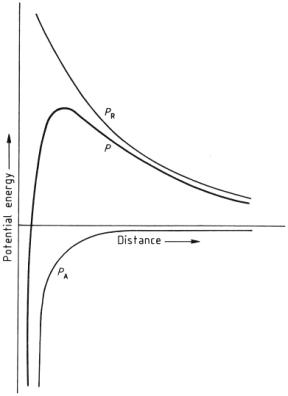
Figure 19: Potential Energy versus Distance
The smaller the potential energy, the easier it is to remove the solid particle. Although a soil already in the washing liquor is less likely to be redeposited on the clothing if the potential barrier is large.
Bi-layers are first created due to attractive Van der Waals forces when no electrical double layer exists. The formation of bi-layer structures causes the free energy of the system to be reduced. As a result of the greater stability of the bi-layer, it takes a considerable amount of energy (mechanical energy) to introduce contact between the lamellar droplet and the substrate. This is important when considering anti-redeposition of the soil.
The presence of calcium ions in solution also is relevant to the potential energy theory. The Schulze-Hardy rule discusses the how water hardness causes the compression of the double layer. At high concentrations the calcium ions may induce more attractive forces causing flocculation, which would lead to less efficient detergency.
In addition to surface potential,
the electrical charge of the surfactant and the fibers of the clothing are also
important. Generally, the soil/pigment and fiber both have has a negative
charge, which can be altered depending on the pH of the solution. The following diagram (Figure 20) shows that
changes in pH and its effect on potential energy of different fabric types.
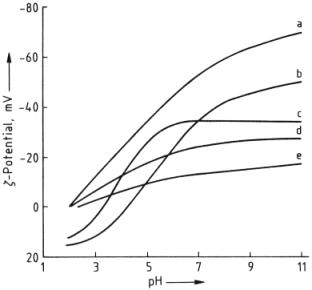
Figure 20: Potential of various fibers as a function of pH [66]
a) Wool; b) Nylon;
c) Silk; d) Cotton; e) Viscose
Although pH can enhance the performance of the detergency it is not good enough to create repulsive forces for steric stabilization. As more and more anionic surfactants are absorbed, the negative charge on the surface increases. Therefore the dispersing power of the pigments is also increased, which results in reduction of redeposition of removed soil.
The most effective soil removal therefore occurs at high surfactant concentrations where there is the greatest negative charge. When the laundry goes through the rinse cycle, charge reversal takes place creating a neutral environment. This environment of electrical neutrality is avoided because it would enhance redeposition of the soil or pigment. For this reason, anionic surfactants are used instead of cationic surfactant for the effective detergency.
Complexing agents show similar effects to anionic surfactants. Complexing agents undergo specific attraction to surfaces, which demonstrate a distinct delocalized charge. The benefit of using complexing agents is that they can be specifically absorbed unlike anionic surfactants, which are absorbed at all hydrophobic surfaces. By adding complexing agents to the washing solution, the absorption of the anionic surfactant on the metal oxides is diminished. Although the opposite is true at surface of the fiber, the absorption is enhanced by the electrolyte nature of the complexing agent. Due to the fact that multiple soil types exist on the surface of the soiled clothing, the specificity of the complexing agent and the surfactant provide complementary functions to the fabrics.
The role of the nonionic surfactant for the removal of particulate soils is similar for other soil types. The nonionic surfactant does not affect the surface charge but enhances surface absorption. The nonionic surfactant shows significant absorption at the hydrophobic surface. The hydration of the nonionic surfactants is important when considering redeposition. As the amount of hydrated spheres that surround the substrate increases, interference is created which reduces Van der Waals forces, thereby reducing redeposition[22].
Calcium-Containing Particulate Soil
Calcium containing particulate soils is common on textile fabric surfaces. The problem with these soils is that they are not very soluble. As the hardness of the water increases, the solubility is minimized. Although this is true, the calcium ions can show some solubility when using distilled water as the washing solvent. This small amount of solubility can cause the break up of the calcium-containing solid on the fabric until the extent of the calcium is absorbed in the washing solution
Types of Fabrics
The type of fabric being washed determines the type of soil removal. One example can be demonstrated between textile fabrics, which contain calcium ions, and synthetic fibers, which have low calcium content. The major difference between these fabrics is their “wettability” due to their degree of hydrophobic and hydrophilic nature.[23] As a result, the complexing agents also react differently with each type of soil. Sodiumtriphosphate, a common complexing agent, shows enhancing effects to the removal of soil from both synthetic and cotton garments. The efficiency is more dependent on the hydrophilic/hydrophobic nature of the fabric. One example is that the effect of sodium triphosphate enhancing the removal of soils from hydrophilic fibers such as poylamide or polyacrylonitrile, but shows minimal effects when considering hydrophobic textile fibers. Although soil removal is specific to the fabric type, these effects can complement each other when considering the detergency of fabric blends in the presence of soil mixture.
Processing
Figure
21: Manufacture of liquid detergent[25]
Packaging
The three main purposes of packaging are to protect the product so that its quality does not change between manufacture and purchase, to supply information about the detergent to the consumer, and to make handling easier. There are many factors companies must consider when packaging liquid laundry detergent. The selection of packaging materials involves taking into account product compatibility, cost, safety, waste, and appeal. Advances in plastics have made the goal of environmentally conscious packaging more easily obtainable. Convenience is also a very important issue. The size and shape of the bottle must be well thought-out so that consumers like the design and want to use the product again. Cheskin Research conducted a telephone survey of 200 people, where each person was asked to rate the importance of certain package features. The following figure shows what consumers notice.
|
Most Useful Package Attributes |
|
Percent Stating
(Sample Size = 200) |
Figure 22: Important package features[26]
Typical bottles for liquid detergent are recyclable plastic. Companies such as Procter & Gamble have been gradually adding recycled material to their plastic in order to cut down on waste. These bottles generally contain 25% recycled plastic. Smaller bottles containing a concentrated detergent solution are very popular. This results in less solid waste because the same amount of laundry can be done with a smaller container. Concentrated detergents use less chemicals per load of wash as well. Another way detergent manufacturers reduce waste is by selling refillable containers. This concept was introduced in the early 1990s. A consumer purchases a larger container initially, and then buys refills that range 65-90% smaller than the original container.
Environmental Considerations
In order to create the most marketable product, the detergent industry must keep up with changing societies. The trend toward more environmentally friendly washing machines has forced detergent manufacturers to adjust and compensate for these changes. New washing machines pose problems because they use less water, energy, and the temperature of the water is lower. If the industry does not meet its past standards for new technologies and concerns, consumers will be dissatisfied.
Water consumption is a major concern worldwide. This needs to be addressed by detergent producers because laundry contributes to water usage. Niall Fitzgerald, chairman of Unilever, voiced his concern: “By 2025, two-thirds of our village (the earth) will be living with “water stress,” meaning that they won’t even have enough for safe drinking water and sanitation-never mind enough for a wash load.”[27] His priorities for the future of detergents include minimizing the amount of water necessary for washing, the ability to wash with poor water quality such as cold water, gray water, and salt water.
Market Sales
There are several types of laundry detergents on the market currently. There are powders, tablets, liquids, and different types of fragrances, pre-treatments, and boosters. The leading style of detergent is liquid, with sales surpassing that of powders for the first time in 1998. According to Information Resources, Inc., Chicago, for the 52 weeks ending on October 7, 2001, $3 billion of liquid detergent was sold, as opposed to $1.8 in powders. The popularity of liquid detergent is attributed to its convenience. Dispensing and measuring the liquid is thought to be much easier than using a cumbersome box of powder. Additionally, liquids dissolve easier in water than powder detergents. Speculators claim powder detergents have a market only because it is cheaper per load of laundry to use powder. The top three manufacturers of liquid laundry detergent have been the same for the past two years. In descending order, they are Procter & Gamble, Lever Brothers, and Dial[28].
The leading brand of liquid detergent is Tide®, and it has held this top position for several years. The sales for the year until October 7, 2001 were $1 billion according to Information Resources, Inc. This figure means that Tide® controls over 33% of the entire liquid laundry detergent market in the United States[29]. Other top brands include All®, Purex®, Wisk®, Xtra®, and Cheer®. There are virtually no middle-tier brands of detergent because consumers either tend to buy for quality (more expensive liquid varieties), or price (inexpensive powders). The following chart shows the market breakdown by brand[30].
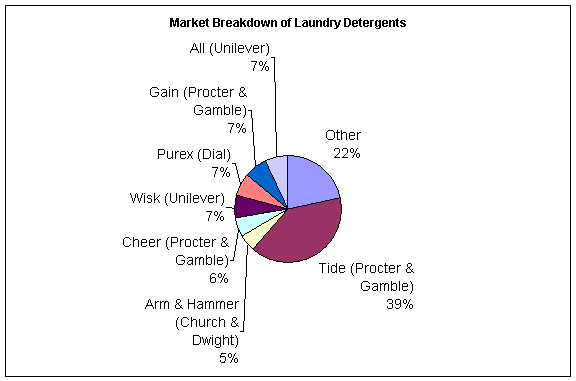
Figure
23: Detergent Market[31]
Endnotes