
Poly(dimethylsiloxane)

Poly(dimethylsiloxane)
Silicon is a Group 4 (IVA) element found in the periodic table beneath carbon, and it is, by far, the most abundant element in the Group 4 elements. Some of its characteristics are similar to carbon, but overall it can be seen as a completely different element. It makes up 27% of the earth's crust by mass and it is second in abundance in the world (after oxygen). Silicon has semi-metallic properties, thus, it is important in the semiconductor industry with wide ranges of applications in computers and solar energy collection. It is very rare to find silicon by itself in nature; it is usually bound to oxygen as either SiO2 or SiO4. Silicon dioxide has many forms found in nature, the most common being quartz, a major constituent of sandstone and granite, as well as being a major component of glass.
Silicon bonding can be compared to carbon bonding in many ways. Carbon is the backbone of life and can form chains of infinite length. Silane, SiH4, and methane, CH4, are both very stable tetrahedral compounds, however as you build chains, the carbon chain is stable but the silane chains stability decreases with length. This is due to many facts; 1) the Si-Si bond is slightly weaker than the C-C bond, 2) the Si-H bond is weaker than the C-H bond, 3) silicon is less electronegative than hydrogen while carbon is more electronegative than hydrogen, and 4) silicon is larger, providing greater surface area, also they have low lying d orbitals, both of which promote nucleophilic attack.
Since silicon oxides are more stable than silicon-silicon bonding, chains are more readily made from silicon oxide and are called siloxanes. There are many ways to make siloxanes including step-growth polymerization (a type of polycondensation) and ring-opening copolymerization.
Step-growth polymerization of linear polysiloxanes include either homocondensations of silanol-ended siloxanes or heterocondensations of silanol-ended species with monomers containing good leaving groups. Both of these reactions are respectable ways of forming polysiloxanes, however homocondensation is more commonly studied.
Homocondensation, also called polycondensation, is a reaction in which two molecules including the silanol group condense to form a polymer. This is illustrated in the reactions below:

This reaction has been determined to have a second order rate law. The study of the kinetics of this reaction by Lasocki and Chrzczonowicz determined that the chemical behavior of the SiOH group at the end of the polymer chain are strongly influenced by two structural effects: an electronic effect acting at a short distance and molecular interactions affecting remote places in the polymer chain (Jones, 60).
The short distance effects cause the condensation reaction to be seen as unusual because the siloxane bond is cleaved rather than the reactive hydroxyl group. In the same way the siloxane might activate the hydroxyl group in a monomer which would explain the high reactivity of the molecule in polycondensation (Jones, 60). These reactions are shown below:

The results, found by theoretical chemists, show that the geometry of the orbitals and their interactions stabilize the silanol structure and strengthens the silicon to hydroxyl group bond (in effect making a partial double bond character).
Since the silanol group is both a good proton donor and acceptor, the molecular interactions between molecules is high. This causes the silanol to silanol hydrogen bond to be readily formed both intermolecular and intramolecular. These interactions can be considered catalysts for polycondensation. For short chains, the silanol end group is a very effective catalyst but with elongation of the chain this becomes a less effective catalyst due to the siloxane chain length (Jones, 71).
Another way of forming polysiloxanes is the ring-opening reaction which form either random or block copolymers. In this reaction the silicon oxygen bond is broken and the chain is extended. The polymerization only happens when there is some of the co-monomer in solution. The lower the amount of this co-monomer, the longer the chain length and the higher the degree of polymerization. A generic equation for this reaction is:

where the initiator, AB, can be either an acid or a base. Some of the initiators are listed below:
Initiators for Cyclosiloxane Polymerization
Basic initiators: GOH, GOSi=, GOR, GR, GSR
Acidic initiators: acid clays, HF, HI3, HCl-FeCl3, H2SO4, CF3SO3H
where G is an alkali metal or a quaternary ammonium or phosphonium group
and R is an alkyl, polystyryl, or poly(trimethylsilylvinyl) (chart
taken from Silicon-Based Polymer Science). When a strong acid
or base is used, the reaction is reversible and the equilibrium
constant is represented as:

Termination of the reaction occurs with neutralization of the acid or the base.
Polysiloxanes are known for their useful properties, such as flexibility, permeability to gases, low glass transition temperature, Tg, (about 146K for poly(dimethylsiloxane)), and low surface energy. Flexibility is an important concept to understand because by modification of polymers, one can get the flexibility or rigidness that is desired. Flexibility also has a role in the glass transition temperature. The higher the flexibility, the lower the glass transition temperature. The flexibility of a chain can be determined by conformational analysis which assesses the isomeric states of the polymer and the energy differences between them. The problem with this sort of experimentation is that it is cumbersome since the length of the chain allows many degrees of freedom, for rotation and movement, and all these degrees have to be studied simultaneously.
Polysiloxanes are so flexible because they show two types of flexibility: torsion flexibility and bending flexibility. Torsion flexibility is the ability of the atoms to rotate around a chemical bond. It considers that the bond length and angles remain unchanged throughout the process. Bending flexibility occurs when there is a large hindrance between non-bonded atoms where there are unfavorable torsion angles. For instance, the Si-O-Si bond is very bendable and can vary between 135 and 180 . On the other hand the O-Si-O bond is rather un-bendable and can only vary between 102 and 112 . These polymers can be made less flexible or more rigid by varying the structure. For instance, combining two polysiloxane chains into a ladder structure, insertion of rigid groups into the structure, or adding bulky side groups will all increase rigidness. Changing the structure will also effect the glass transition temperature; bulky groups that hinder the flexibility will also raise the Tg.
Polysiloxanes also have a much higher permeability to gases than other polymers. One experiment that has been done is to increase the selectivity of the gas without decreasing the permeability. This is rather hard to do since increasing the selectivity will invariably decrease the selectivity. The permeability of polysiloxane allows these polymers to be used as membranes where gas needs to be exchanged across the membrane but other molecules (such as bacteria) need to be excluded, for example in artificial skin and soft contacts.
One polymer derivative of siloxane that has unusually high permeability
is poly(dimethylsiloxane) or PDMS. PDMS also has other unusual
properties. These include: low internal pressure, low bulk
viscosity, low temperature coefficient of 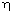 , low entropy of dilution,
and low excess volume upon mixing. There are many theories that have
been suggested to explain these characteristics but nothing has been
proven. One suggestion involves low intermolecular interactions and
high rotational and oscillatory freedom of the methyl side groups.
Another focuses on the chain's irregular cross section, which is
large at the substituted silicon atom and small at the unsubstituted
oxygen atom.
, low entropy of dilution,
and low excess volume upon mixing. There are many theories that have
been suggested to explain these characteristics but nothing has been
proven. One suggestion involves low intermolecular interactions and
high rotational and oscillatory freedom of the methyl side groups.
Another focuses on the chain's irregular cross section, which is
large at the substituted silicon atom and small at the unsubstituted
oxygen atom.
Low surface energy, or surface tension, is another key feature of polysiloxanes. The surface energy can be measured by various techniques, including a measure of the contact angles between the polymer and water and by XPS instrumentation. The reason for the surface energy being so low is that the methyl groups have virtually no interactions with each other. Another factor in the surface energy is the boiling point. Since it is low for polysiloxanes, the surface energy is low as well.
With all the different characteristics of polysiloxanes come a variety of applications, both medical and non-medical. Since a lot of favorable characteristics are known and usable, this polymer is a popular candidate for various purposes. The medical applications include prostheses, artificial organs, facial reconstruction, catheters, artificial skin, contact lenses, and drug delivery systems while the non-medical applications include high-performance elastomers, membranes, electrical insulators, water repellants, anti-foaming agents, mold release agents, adhesives and protective coatings, release control agents for agricultural chemicals, and hydraulic, heat-transfer, and dielectric fluids.
One of the medical applications listed above is contact lenses. Sometimes the soft contact lenses are made of PDMS because of the high permeability of gas molecules. The eye requires oxygen for the metabolic processes and it is acquired by the diffusion of air rather than from internal blood vessels. PDMS is optimal for this use because of the high gas permeability. Unfortunately the polymer is too hydrophobic to be wetted by the tears and this property limits comfort. The remedy for this problem is to cover the polymer with a thin layer of a hydrophilic polymer to maximize comfort. This hydrophilic layer does not affect the permeability because of its thinness. Artificial skin and facial reconstruction works similarly to the contacts. Since the polymer is flexible and gas permeable then it moves with bodily movements, and it is permeable to gases but not to bacteria and other substances that one would not want in their body.
Polysiloxanes are good for non-medical applications as well. For instance, the polymer is hydrophobic and is a good water repellant, as well as being slippery so other substances will not stick to it either. Also, since it is permeable to gases while being impermeable to particles, it is a good protective coating. The bonding is strong and so the polymer can be used as a good adhesive as well. These three applications are also enhanced because of the flexibility of the polymer. Since it has the ability to bend and twist, there wont be as much cracking and chipping going on in the application.
Zeigler, John M. and Fearon, F. W. Gordon. Silicon-Based Polymer
Science. The American Chemical Society; Washington DC 1990.

CE435- Introduction to Polymers