
Polyethylene
CE 435 - Introduction to Polymers
Term Project
Brian Aylward
Anthony J. Kurek Jr.
Kevin Todtenhagen
4 / 29 / 1999
Introduction
We will begin our description of the polymer, polyethylene, with an informative introduction, which will provide a brief description of the origins of thermoplastic polymers, as well as the origins polyethylene. This introduction will show some advancements in polymer technology that lead to the need for the development of new and better polymers. These new and better polymers, including polyethylene, were used to sustain the development of more new technologies in the polymer industry. Also this introduction will show how the discovery of polyethylene aided in the development of the polymer industry.
History and Advancements of Thermoplastic Polymers
The uses of naturally occurring polymers, including tortoise shell and horn, are referenced throughout history. These compounds are natural cellulose materials that are produced by many animals. These compounds are unique because they possess special processing capabilities. The most important capability of these polymers is the fact that they can be heated to a softening temperature, and while at this temperature the material can be formed to a desired shape, and once the material is allowed to cool the desired shape is retained. The early uses of these materials have been shown as household utensils, including spoons, goblets, and bowls. These discoveries can be called the beginnings of thermoplastic polymers.
The first non-naturally occurring thermoplastic polymer was discovered and produced in 1868, by John Wesley Hyatt. Hyatt was looking for a synthetic material to replace the ivory used in piano keys and billiard balls. This was needed because about this time there was a shortage of natural ivory. It was about this time when many governments had restricted the import of the ivory materials. Many manufacturers while looking for replacements for these materials held public contests to find cheap replacements for the natural materials. This was the case for Hyatt, who was a journalist by trade. His material given the trade name 'Celluloid' allowed the technology used on the early naturally occurring polymers to be used in processing. The only problem with this material was that there was a thermal instability when the temperature of the material was increased significantly. This thermal instability led the search for better polymers.
In the early 1870's another cellulose derivative, Cellulose Acetate, was discovered. This new polymer had many of the same properties of the Celluloid material discovered by Hyatt. This material however did not possess the same thermal instability that the Celluloid polymer. Because of this fact the development of several thermal-processing technologies developed. Included among these technologies was injection molding. With the discovery and development of the Cellulose Acetate polymer, molding industries were allowed develop and prosper in the early 1900's.
The next significant advancement in polymer technology came with the introduction of polystyrene. It was possible for Polystyrene polymer to be processed in the same methods as those early cellulose polymers. This polymer was allowed to prosper in the late 1920's and throughout the end of WWII. This was because a by-product of the U.S. Military's Rubber Program, which was in production before and during WWII. It was this governmental program that made the styrene monomer to be readily available and probably most important it was cheap. This caused many manufactures to get into the polystyrene processing. The toy industry was a major purchaser of polystyrene. Polystyrene polymer had one major disadvantage, which was the fact that it was brittle.
These early polymers aided in the development of many polymer technologies. Each of those early polymers had their disadvantages, and some of these were discussed above. Due to the huge amount of polystyrene introduced into the public a general dissatisfaction with the plastics industry as a whole. The market was ready for more durable polymers.
Ethylene has been used in chemical reactions for as long as it has been in existence. It is a very useful and reactive substance due to its double bond. It was not until 1898 however that the first experiments to create long chain molecules with ethylene had commenced. These first reactions however had several problems. First, solid polyethylene had not been able to be produced. That is all the products of these early reactions had been waxes and greases. These non-solid polyethylene polymers had little use at the time.
It wasn't until 1933 when the first solid polyethylene polymer had been produced. Two scientists working for the Imperial Chemical Company had made this discovery. These scientists, E. W. Fawcett and R. O. Gibson, had produced this solid polymer while experimenting with pure ethylene gas at extremely high pressure and temperature. The ICI didn't wait long before obtaining a patent for this new material and began to market it.
The first applications of this early polyethylene polymer were for use as coatings. The primary coating that the ICI marketed polyethylene for was as insulation on electrical wires. The Telegraph Construction Maintenance Company had invested a lot in order to coat their submarine cables with this new polymer. It was about this time when the British Military had begun to use this polymer in the coating of their high frequency cabling and wires. This allowed the British to make great technological strides in the radar field. History has stated that it was because of the polyethylene polymer used here that the British had possessed the best radar capabilities in the world.
It was these military advancements by the British that interested many of the world powers at the time. Included among these powers was the U.S. government. The U.S. government was then able to persuaded two American based companies (Dupont Corp. and Union Carbide Corp.) to seek licenses from the ICI so that the production of polyethylene could begin stateside. Before the end of the Second World War the production of U.S. polyethylene had surpassed that made by Britain.
It was not until after WWII when extensive chemical studies began to be performed on the polyethylene polymer. The manufacturers of the polymer quickly marketed these new properties. New methods for processing polyethylene had been discovered during these physical tests. Film extrusion and injection molding were two of the new processing techniques used on polyethylene after WWII.
Another important discovery concerning polyethylene polymers came at about the 1940's. It was about this time when the IR spectroscopy technology had begun to develop. Substances including polymers were often subjected to this test to discover the compound's structure. An important discovery that was made when polyethylene was subjected to this test had shown that there were more than two methyl groups present in a single polyethylene chain. This lead to the natural conclusion the polyethylene polymer was not a straight chain polymer as previously thought, for this spectroscopic result to occur the polyethylene polymer must contain branches along its carbon backbone.
It was also about this time when work commenced on discovering ways of lowering the reaction conditions to produce polyethylene polymers. A polyethylene polymerization mechanism were the reaction conditions were lowered was accomplished in 1951 by Karl Ziegler. Ziegler was able to produce the solid polyethylene polymer at low pressures and temperatures by employing a catalyst. Testing on Ziegler's polyethylene polymer had shown that it had a larger density then the original high-pressure polyethylene process. It was after this discovery that characterization of polyethylene polymers were made on their density.
Shortly after Ziegler had discovered HDPE (High Density Polyethylene) another group of researchers working at the Phillips Petroleum Inc. had discovered other catalysts that produced the same results as Ziegler's process. These HDPE polymers were characterized as having fewer branches than those early high-pressure polyethylene polymers (LDPE - Low Density Polyethylene).
In 1977 researchers working at Union Carbide Corporation discovered a method to produce a new polyethylene. This new polyethylene was made from monomers other than ethylene gases, and as a result contained few short branches. Because of this property they were termed as LLDPE (Linear Low-Density Polyethylene) Polymers.
New Polyethylene polymers are still being developed today. This polymer contains a rich history rooted back more than a century ago. We will now present the individual polyethylene polymers giving summaries of the reaction chemistry and physical/mechanical properties of each type of polyethylene polymer.
Ethylene Monomer
General Properties - reactivity due to double bond
Manufacture of Ethylene - 90% of production of commercial grade Ethylene Gas is recovered from natural and refinery gases
Types of Polyethylene Polymers
Production of LDPE
LDPE was the first solid polyethylene polymer produced. It was first produced in 1933 in the research laboratories of the ICI in England. The two scientists accredited for the development of this new polymer were E. W. Fawcett and R. O. Gibson. These two scientists were able to produce this polyethylene polymer only at extremely high pressure and temperature using pure ethylene gas. Unfortunately, ethylene gas doesn't react on its own at high temperature or high pressure. These two scientists needed to inject a small amount of oxygen gas into the reaction vessel in order to cause a reaction. The reaction they discovered was very rapid and also quite exothermic. The thermoplastic resin they formed in this reaction was noticed to have very remarkable and different properties from the early polyethylene waxes and pastes. The typical results of this method and the earlier methods can be shown below(2):

After some study by ICI scientists, it was determined that the high-pressure reaction to form polyethylene followed a Free Radical Polymerization mechanism. This classical polymerization mechanism is one that follows the three main steps including; initiation, propagation, and termination. The LDPE mechanism is described below in detail:
The initiation and propagation steps in this reaction are as follows(3):



Controlling the Properties of Polyethylene
Why would changing the reaction conditions have an effect on the properties of the LDPE polymers? It was in the 1940's when Infer Red Spectrum analysis of chemical compounds began to appear as an important method for determination of chemical structure. Polyethylene, as shown above can have different properties depending on the reaction conditions. When the polyethylene polymers were subjected to IR Spectroscopy the results were startling. The results of the testing had shown that the polymer contained a large number of methyl groups in a singe molecule. These results are explained because the molecule has many branches of varying length along its backbone chain. The amount of chains and length of the chains are what varies the properties of the polymer. Branching in the polymer must then be a function of pressure. In fact as the reaction pressure is increased the branching in the polymer is decreased.
What causes the branching in the polyethylene polymers? The branching can be explained through one of the two types of chain transfer. Short chains consist of branches with lengths varying from 1 to 3 carbon atoms. These short carbon branches can exist along the backbone of the polyethylene molecule in numbers from 10 to 60 branches per 1000 backbone carbons. Intermolecular chain transfer explains the creation of the short branches in the polyethylene polymer. See the below picture(3). There are not only short branches along the carbon backbone of the polyethylene. The long chain branches present in the polyethylene have a length longer than 5 carbon atoms. These branches can be present in the polymer in numbers of 1 to 4 branches per 1000 backbone carbon atoms. The cause of the long chain branches intermolecular chain transfer. See the below picture(3):

Since one can control the properties of the polyethylene polymer, methods of controlling the branching amount in the polymer had to be developed. Two commercial methods for controlling the amount of branching in the polymer that were developed for the manufacture of polyethylene are based on the reactor type. The two commercial reactor methods to produce the polyethylene are using an autoclave reactor or a tubular reactor. The design of the reactor has an influence on the amount of intermolecular chain transfer (which is the important type of chain transfer in this reaction). Tubular reactors typically operate at pressures of 2000atm and 350 degrees Celsius, and the typical conversion rates for this type of reactor are 15-20 percent. Autoclave reactors typically operate at pressures of 1400atm and 250 degrees Celsius, and the typical conversion rates for this type of reactor are 16-23 percent. The results of the reactor type methods are as follows(3):

High Density Polyethylene
Up until the 1950’s the only type of polyethylene produced was low-density polyethylene. Low-Density polyethylene was being produced at extremely high pressures. This high-pressure polymerization created polyethylene with many branches; the branches are created due to intermolecular and intramolecular chain transfer during polymerization. The mechanism for the polymerization of low-density polyethylene is free radical polymerization. The uses of low-density polyethylene are limited due to high number of branches. Because of the extreme pressure needed to create low density polyethylene and its limited uses, Karl Ziegler was trying to create polyethylene at atmospheric pressure. Karl Ziegler, a German scientist, made the greatest contribution to producing high-density polyethylene.
The difference in high-density polyethylene and low-density polyethylene is the degree of branching. The mechanical properties change drastically when comparing high-density polyethylene to low density polyethylene. In general, the degree of branching in polyethylene determines its mechanical properties. For example, high-density polyethylene is more crystalline than low-density polyethylene because it contains fewer branches. Unlike low-density polyethylene, Karl Ziegler created polyethylene with the use of a catalyst at atmospheric pressure. However, at first, this process did not go very smoothly. At first, Ziegler reacted Aluminum triethyl, a metal alkyl, with ethylene gas at atmospheric pressure. The reaction only yielded polyethylene with a molecular weight of 4,000. The reason for this is due to the displacement reaction where the aluminum-carbon bond displaces into a double bond.
Ziegler realized the problem was due to the reactivity of aluminum triethyl, as shown in the reaction above. Because of this, Ziegler reacted aluminum triethyl, a metal alkyl, with titanium tetrachloride, an organometallic. He hoped the reaction of the two compounds would create an active site where polymerization would occur. When the two compounds were placed in a reactive vessel the precipitate titanium trichloride forms along with small amounts of unreacted aluminum triethyl. The titanium trichloride has a lower valence state than titanium tetrachloride, thus, making it more reactive than titanium tetrachloride in the presence of the monomer ethylene. When ethylene was introduced to the precipitate along with an inert solvent, polyethylene with a high molecular and very little branching was formed. This polymerization took place at atmospheric pressure and 100° Celsius.
How did this polymerization occur? The question is still debated today. The mechanism of the reaction to create high-density polyethylene is still not fully understood today. The question that persists is where the active site of the polymerization takes place. Today, the theory put forth by Natta is most credibly believed. Natta worked alongside Ziegler in a laboratory in Germany. He created polypropylene by reacting an organometallic with a metal alkyl to create an active site for polymerization. After creating the catalyst, Natta introduced propylene to create polypropylene. The catalyst created is very similar to the catalyst Ziegler created to produce high-density polyethylene. Because of this, the catalyst’s used today to create polyethylene and other types of polymers are referred to as the Ziegler-Natta catalyst. Natta explained the mechanism by explaining that the reactive site of the catalyst is the Ti-C bond and not the Al-C bond formed during the initiation step. The following reactions show the initiation step that creates the active site for polymerization, and the propagation step for the production of polyethylene(1):
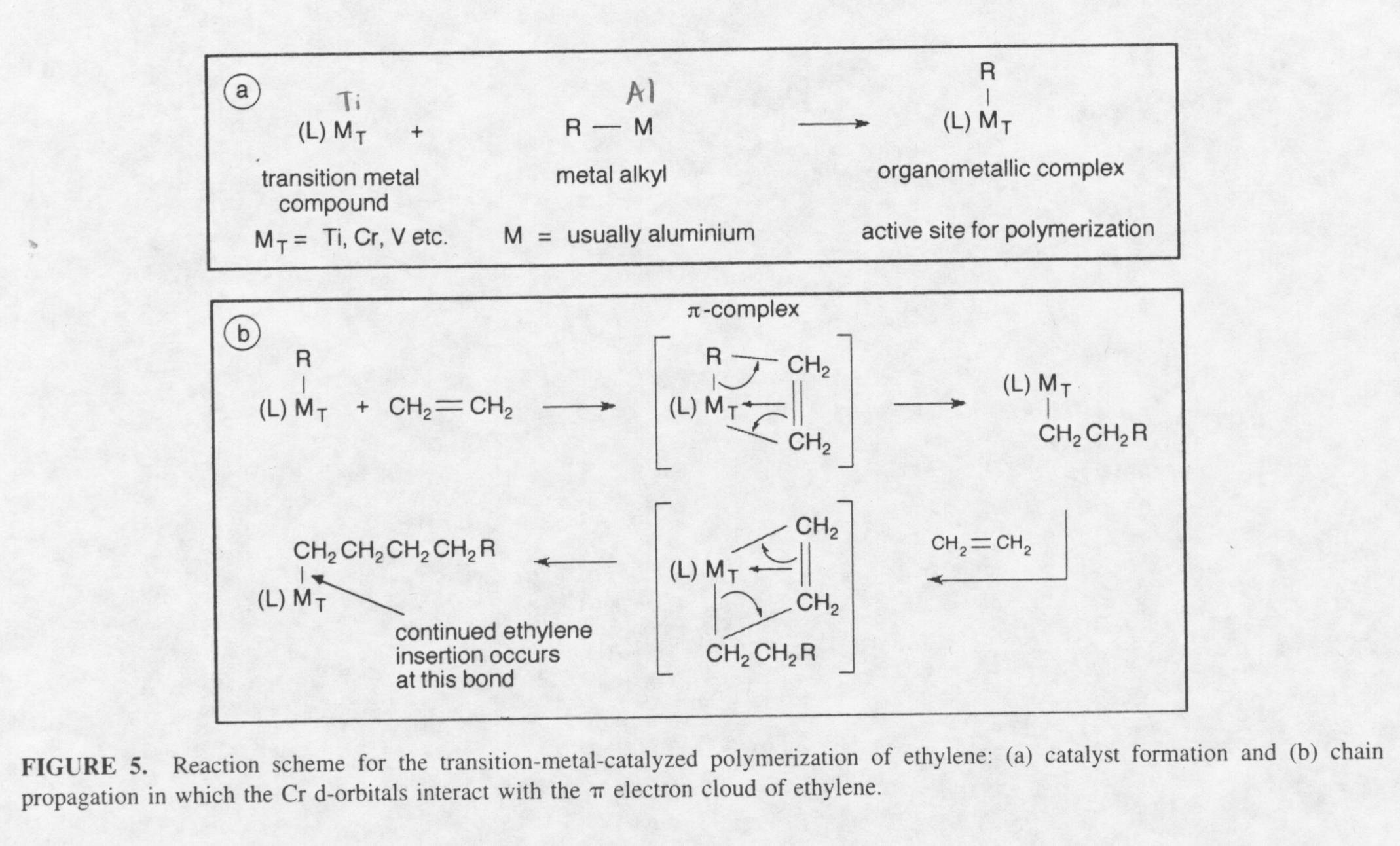
The idea of the mechanism involves catalyst formation and chain propagation in which the Chromium d-orbitals interact with the p electron cloud of ethylene. The mechanism is classified as anionic polymerization or "living" polymerization. Living polymerization means the polymerization continues until the concentration of ethylene runs out. Because of this, the molecular weight of the polyethylene created can be extremely high. One way to control the molecular weight of high-density polyethylene created is through chain transfer reagents. Some typical chain transfer reactions are shown below:
Chain Transfer with monomer:
![]()
This reaction occurs with some heterogeneous Ziegler-Natta catalysts
Transfer with organometallic compounds:
![]()
This reaction leaves aluminum bound to the polymer chain. Exposure to moisture hydrolyzes the bond.
Chain transfer with hydrogen:
![]()
The reaction most important to controlling the molecular weight of high-density polyethylene created by the Ziegler Process is chain transfer with the introduction of hydrogen.
The production of high-density polyethylene from the Ziegler process is shown below(2):
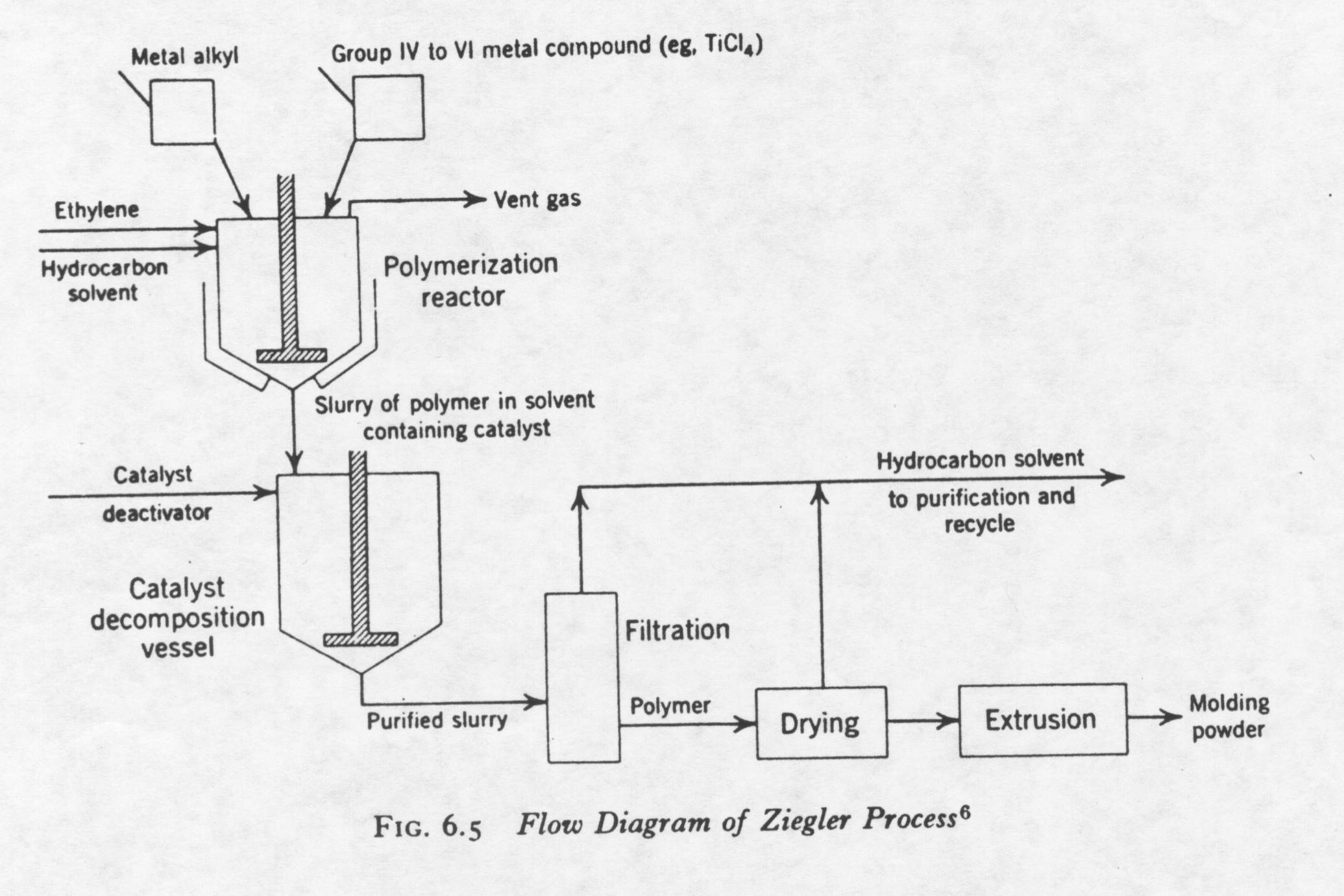
A brief description of the Ziegler process will be explained in the following paragraph. First, the organometallic compound (i.e. titanium tetrachloride) is reacted in a reaction vessel with a metal alkyl at a temperature between 100-130 degrees Celsius in the presence of a solvent. The pressure of the reaction vessel is between atmospheric and 20 atm. Ethylene is introduced into the reactor vessel in the gas phase. The boiling point of ethylene is approximately –100 degrees Celsius. The ethylene reacts with the active site of the catalyst to produce polyethylene. The solvent is used to dissipate heat. The solvent must not vaporize or react with any of the compounds in the reactor (inert solvent). The melting point of high-density polyethylene is approximately 130 degrees Celsius. Therefore, the polyethylene formed is in the solid phase. This type of polymerization is called slurry polymerization or suspension polymerization. The slurry solution is passed to a catalyst decomposition bed where the catalyst is deactivated. The catalyst is not completely used in the polymerization process. Catalyst decomposition is achieved with the addition of an alcohol. Polyethylene is then recovered with the extraction of the solvent, and a filtration and drying process. The polyethylene can then be processed and manufactured. The polyethylene created by the Ziegler process has a molecular weight 20,000 and 1.5 million. The molecular weight is controlled in a number of different ways: pressure of the reactor vessel (higher pressure, less branches), temperature in preparation of catalyst (too high of temperature deactivates catalyst), chain transfer reagents, and the ratio of Al/Ti catalyst added to reactor. The table below shows the weight average for different Al/Ti ratios for the Ziegler process at atmospheric pressure(2):
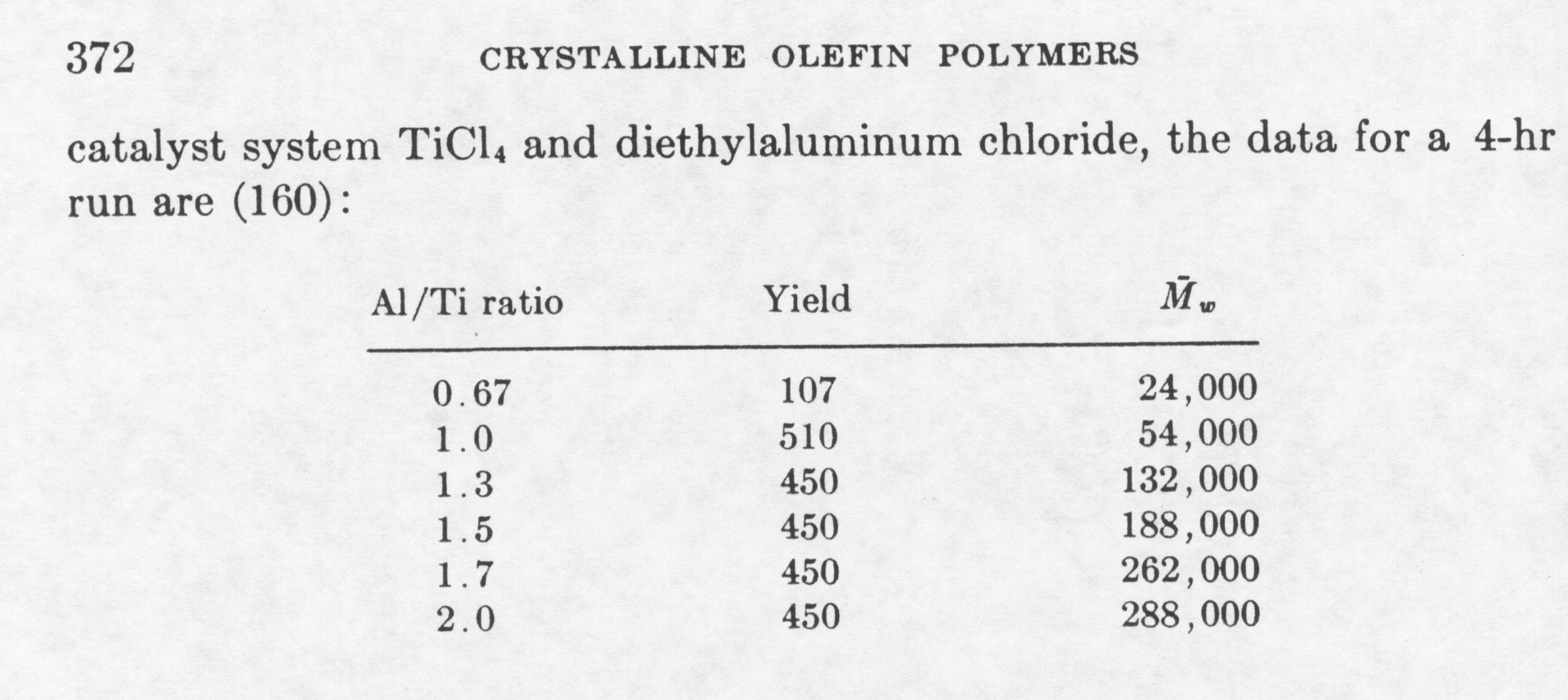
The results show the greatest yield is when the ratio is when the Al/Ti ratio is 0.9. The molecular weight continues to rise over an Al/Ti ratio of 2.0, however, the weight average remains constant. The Ziegler process produced a high-density polyethylene at pressures as low as atmospheric pressure. High-density polyethylene is a more durable polymer when compared to low-density polyethylene due to its lower degree of branching.
Phillips Process
The Phillips process is very similar to the Ziegler Process. The Phillips Process, the process commercialized by Phillips Petroleum Corporation in 1961, uses a catalyst to create an active site for polymerization. Historically, this was the first method used for commercial ethylene polymerization with the original Ziegler catalyst. At present, Phillips Petroleum utilizes a highly active catalyst, chromium oxide on a high-surface area silica, to produce high-density polyethylene. The active site for polymerization, Cr-C bond, is achieved by reacting the catalyst with an olefin. The olefin reduces the valence state of the transition-metal atoms, thus, making it more reactive. The reaction mechanism is similar to the mechanism explained for the Zeigler process. The mechanism is classified as anionic polymerization or "living" polymerization.
The difference in today’s process to produce high-density polyethylene versus the Ziegler process is a result of the catalyst used. The first generation Ziegler catalysts were not very active and had to be removed through a complex extraction process. An alcohol was added to deactivate the catalyst. Many of the polymer processes had in front of the main reactor a special reactor in which the catalyst preparation took place and the viscosity, morphology, control was a very important step. The use of a silica base eliminates this problem. The catalyst is very active and it no longer needs to be removed because all of the catalyst is reacted with the monomer ethylene. The active sites on the monomer are equally accessible to the monomer throughout the particle. Therefore, the polymer chains grow not only outwards but also inwards, causing the granule to expand progressively. The polymer particle will be a replica of the catalyst particle if the mechanical strength of the particle is high enough. Because of the complexity and importance of the silica base catalyst, the catalyst is often prepared in a separate production plant. The Phillips process is shown below(2):
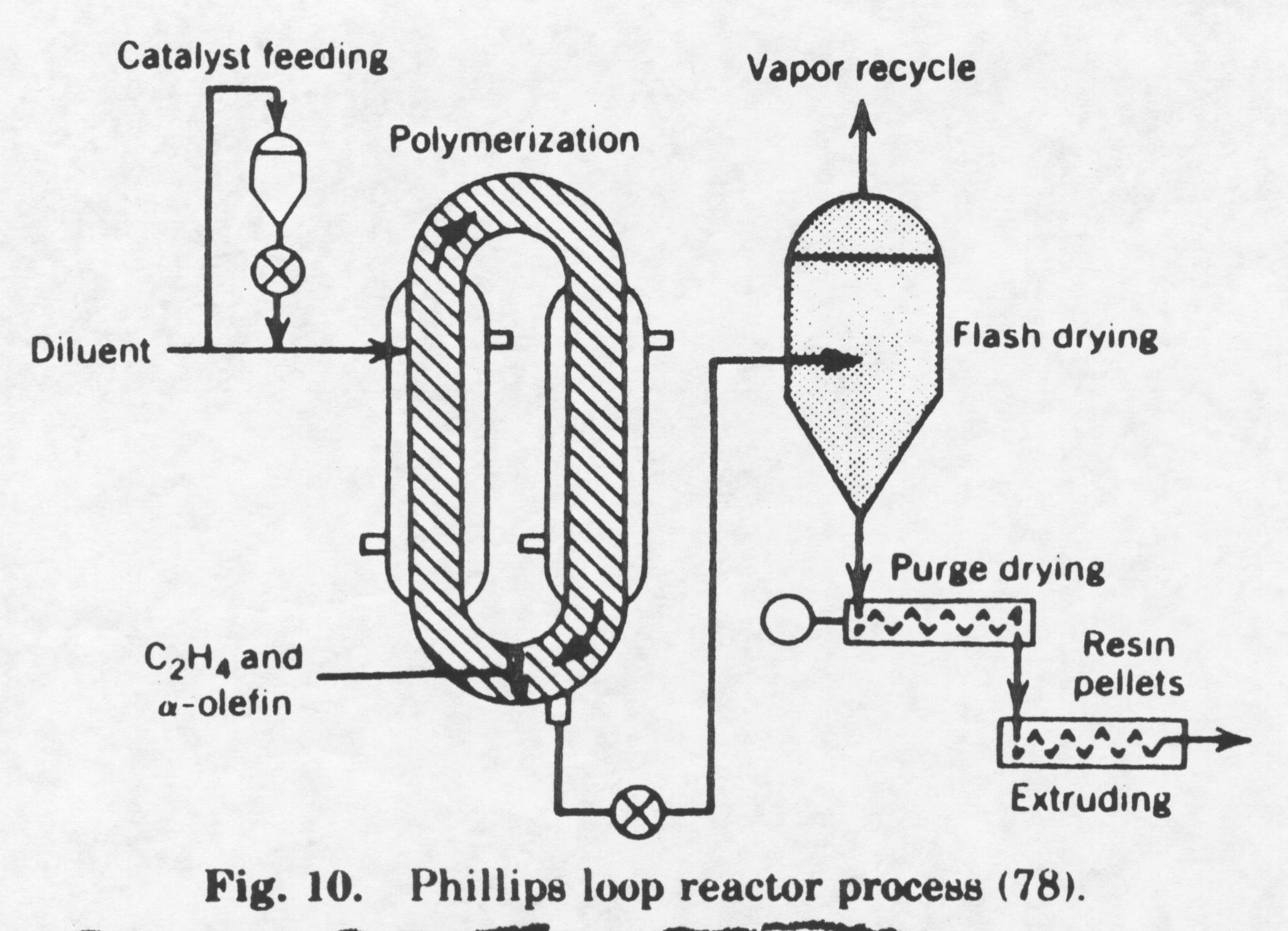
A brief explanation of the Phillips process is provided in the following paragraph. The process shown above represents Phillips Petroleum Co. suspension ethylene polymerization in 1961. The polymer particles are suspended in an inert hydrocarbon. The melting point of high-density polyethylene is approximately 135° Celsius. Therefore, slurry polymerization takes place at a temperature below 135° Celsius; the polymer formed is in the solid state. If the polymerization were to take place at a temperature greater than its melting temperature then the polymer formed would be in the liquid phase. The Phillips process takes place at a temperature between 85-110° Celsius. A loop reactor is used in a liquid-phase process. The catalyst and the inert solvent are introduced into the loop reactor where ethylene and an a -olefin are circulating. The inert solvent is used to dissipate heat as the reaction is highly exothermic. A cooling jacket is also used to dissipate heat. The active sites on the catalyst are equally accessible to the monomer throughout the particle. Therefore, the polymer chains grow not only outwards but also inwards, causing the granule to expand progressively. The reactor consists of a folded loop containing four long runs of pipe 1 m in diameter, connected by short horizontal lengths of 5m. The slurry of HDPE and catalyst particles circulates through the loop at a velocity between 5-12m/s. The reason for the high velocity is because at lower velocities the slurry will deposit on the walls of the reactor causing fouling. The concentration of polymer products in the slurry is 25% by weight. Ethylene, alpha olefin comonomer (if used), an inert solvent, and catalyst components are continuously charged into the reactor at a total pressure of 450 psig. The pressure is a lot higher than the pressure used to create high-density polyethylene by the Ziegler process. The high pressure creates HDPE polyethylene with fewer branches than the HDPE created by the Ziegler process. The HDPE created by the Phillips process typically has one ethyl branch per every 100 molecule chains while HDPE created by the Ziegler process has three ethyl branches per every 100 molecule chains. Because of this, the density of high-density polyethylene created by the Phillips process is higher. This has its advantages in processing. The HDPE created by the Phillips process is more crystalline and it is used to create more durable products. The polymer is concentrated in settling legs to about 60% by weight slurry and continuously removed. The solvent is recovered by hot flashing. The polymer is dried and pelletized. The conversion of ethylene to polyethylene is very high (95%-98%), eliminating ethylene recovery. The molecular weight of high-density polyethylene is again controlled by the temperature of catalyst preparation (too high of temperature increases spontaneous chain transfer, but increases the rate of reaction). The goal of the engineer is to find the temperature that optimizes the process. The molecular weight can be controlled by the addition of hydrogen into the reactor. Chain transfer will then occur. The following chain transfer reactions possibly could take place in the production of HDPE by the Phillips process:
Spontaneous Chain Transfer:
![]()
This is the principal chain-transfer reaction for the Phillips process with the use of a chromium catalyst. Its importance increases rapidly with increasing temperature and it provides easy means of controlling molecular weight.
Chain Transfer with hydrogen:
![]()
This type of chain transfer is not common for controlling the molecular weight for the Phillips process.
The Phillips process creates HDPE with fewer branches than the HDPE created by the Ziegler process. The use of a silica-based catalysts greatly reduces recovery and deactivation time. There are many different companies that produce HDPE today. They all use the idea of a silica-based catalyst and slurry polymerization. The reason why slurry polymerization is used is because solution polymerization has many disadvantages. The polymer created is in the liquid phase; only one phase is present. The polymer created is very viscous and for this reason a high molecular weight polyethylene can not be created by solution polymerization. Some companies use this process, but it is not very common. Another type of polymerization used to create HDPE is gas-phase polymerization. The process involves the reaction an a -olefin with an active catalyst, typically chromium based catalyst that is silica-supported, to create HDPE. This process is most commonly used to produce LLDPE and the molecular weight is controlled with the addition of the chain transfer agent hydrogen. The conversion rate for the production of polyethylene is very high for the Phillips process. The two processes explained above are only two ways to produce HDPE. The Ziegler process was the first method to create HDPE. The method of production of HDPE today using slurry polymerization is very similar to the Ziegler process. The only difference is that companies optimized the process to create a more efficient catalyst, a better way control the molecular weight, and a higher conversion rate of the monomer ethylene to polyethylene. The Phillips Petroleum Company is only company out of a number that optimized the Ziegler process to create HDPE in the most economical manner.
Polyethylene Economics
Comparison in production between 1997 and 1998
(6)
Global effects on polyethylene prices
Economic depression, increase production causing U.S. surplus of LLDPE and HDPE.
Crude oil prices effect price of ethylene.
Economic Forecasts
Polyethylene packaging industry will grow at the fastest rate
Rigid containers and flexible plastic films
Plastic versus paper at the grocery store
 (6)
(6)
References
(1)Allison, J.B., Raff, R. A. F.; "Polyethylene"; Interscience Publishers LTD.; New York; 1956.
(2)Miller, S. A.; "Ethylene and it's Industrial Derivatives"; Benn Limiting; London; 1969.
(3)Colvin, R.; "Polyethylene"; Modern Plastics; January 1999; page 52.
(4)Fried, J. R.; "Polymer Science and Technology"; Prentice Hall; New Jersey; 1995.
(5)Kresser, T.; "Polyethylene"; Reinhold Publishing Corp.; New York; 1961.
(6)Leaversuch, R. D.; "Resin Supply"; Modern Plastics; January 1999; pages 49-51.
(7)Levowitz, I. L.; "Polyethylene"; Modern Plastics; November 1998; page B-3.