
Superabsorbent Polymers

Erin Pytlik
Daryl Molino
Joseph Moritz
INTRODUCTION
Superabsorbent Polymers (SAP):
Superabsorbent polymers are primarily used as an absorbent for water and aqueous solutions for diapers, adult incontinence products, feminine hygiene products, and similar applications. Undoubtedly, in these applications, superabsorbent materials will replace traditional absorbent materials such as cloth, cotton, paper wadding, and cellulose fiber.
Commercial production of superabsorbent polymers began in Japan in 1978, for use in feminine napkins. This early superabsorbent was a crosslinked starch-g-polyacrylate. Polyacrylic acids eventually replaced earlier superabsorbents, and is the primary polymer employed for superabsorbent polymers today.1 In 1980, European countries further developed the superabsorbent polymer for use in baby diapers. This first diapers employing this technology used only a small amount of polymer, approximately 1-2 g. In 1983, a thinner diaper using 4-5 grams of polymer and less fluff was marketed in Japan.
The use of superabsorbent polymers revolutionized the diaper industry. Diaper manufacturers began to design diapers to take advantage of the amazing liquid retention ability of the polymer. Superabsorbent polymers absorb, and retain under a slight mechanical pressure, about 30 times their weight in urine.2 The swollen gel holds the liquid in a solid, rubbery state and prevents the liquid from leaking onto the baby’s skin and clothing.1
Superabsorbent polymers are prepared from acrylic acid and a crosslinker by solution or suspension polymerization. The type and quantity of crosslinker control both the swelling capacity and gel modulus.2 The synthesis and use of crosslinked polyacrylate superabsorbents have been a popular topic in the polymer literature. However, very little information about manufacturing processes has been given due to its proprietary content.1
The properties of superabsorbent polymers can be employed in many different applications. The largest use of superabsorbent polymers is in personal hygiene products. These consumer products include, in order of volume of superabsorbents used, disposable infant diapers, children’s training pants, adult incontinence articles, and feminine sanitary napkins.1 AMCOL estimated their total superabsorbent polymers sold in 1995 to be represented by the graph below: 3
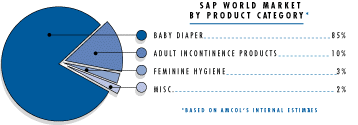
(source: AMCOL website)
MARKET
Demand:
Since the introduction of superabsorbent diapers in Japan in 1983, the global market for superabsorbent polymers has grown and changed dramatically in the last ten years as superabsorbents have replaced fluff pulp in diapers and other personal hygiene articles. Worldwide superabsorbent polymer production capacity grew from only a few million metric tons in 1985 to greater than 700 million tons in 1995 with the United States accounting for 30% of this superabsorbent polymer demand.2
Diaper Manufacturers:
Approximately 75% of the superabsorbent polymers used worldwide are sold in diaper products from five major companies. These manufactures include Proctor & Gamble (P&G), Kimberly-Clark, and other diaper manufacturers such as Paragon Trade Brands, Molnycke, and Unicharm.2
In the United States, Proctor & Gamble is a well-known diaper manufacturer, which produces the popular Pampers diaper. The superabsorbent polymer used in the Pampers diaper holds approximately thirty times its own weight in body fluid.4 The P&G Corporation developed a unique three-piece construction diaper to absorb the moisture and distribute it evenly. The transmission of fluid to the absorbent core allows the fluid to be engulfed, therefore not passing it back to the skin. P&G diapers are now sold in more than 80 countries worldwide with $4 billion in sales.4
Superabsorbent Polymer Manufacturers:
In just twenty years, worldwide production of superabsorbent polymers is in full swing. Many industrial leading countries have companies producing some type of superabsorbent polymer. In the U.S., current manufacturers of acrylate-based superabsorbents include The Dow Chemical Company, Sanyo Chemical Industries, Nippon Shokubai Company, and the Chemdal Corporation, which is a subsidiary of AMCOL International. Other manufacturers located in Europe include AMCOL, Stockhausen GMBH, Dow Chemical, Hoechst Casella, Allied Colloids, and Nippon Shokubai. Superabsorbent polymer production in Japan comes from companies such as Nippon Shokubai, Sanyo, Mitsubishi Petrochemical Company, and Sumitomo Seika.
The leading producers of SAP consist of Stockhausen GMBH, Nippon Shokubai, Chemdal Corporation, Hoechst Casella, Dow Chemical, and Sanyo. These companies manufacture eighty percent of the worldwide production of superabsorbents.
The following table shows SAP production for these industry leaders:2
Stockhausen GMBH 162,000
Nippon Shokubai 137,000
Chemdal Corporation 120,000
Hoechst Casella 94,000
Dow Chemical 90,000
Sanyo 47,000 (metric tons)
Information regarding manufacturing processes of SAP is hard to attain. The majority of manufacturing processes producing SAP employ solution polymerization, in which the monomer acrylic acid is dissolved in a solvent with free radical initiators.1 Another process used to produce SAP is suspension polymerization, although in much smaller quantities. In 1996, there were three commercial superabsorbent polymer manufacturers using a suspension process. These were Kao Soap Co. and Sumitomo Seika in Japan and Elf Atochem S.A. in France.1 Suspension polymerization is a process in which droplets of monomer or monomer solution are dispersed in an immiscible continuous phase. The polymerization is carried out independently in these dispersed droplets.5 Sumitomo Seika and has been the most prominent users of a suspension process to make superabsorbent polymers.
DIAPER CONSTRUCTION
Overall Construction:
Disposable diapers have changed greatly during the past 30 years, but three basic design components are used in any diaper. A diaper consists of an absorbent core between a porous top-sheet and an impermeable back sheet. The top-sheet must do three things: it must allow the urine to flow through it, keep the liquid away from the baby’s skin, and retain the structural integrity of the absorbent core. Usually it is made of a porous, hydrophobic substance, for example, polyester or polypropylene non-woven fabric.1 The back sheet helps keep the baby’s clothing dry and is a nonporous, hydrophobic substance, such as a polyethylene film. The absorbent core takes in the liquid, distributes it to all regions of the core, and holds the liquid under pressure from the baby.
Cost Analysis:
AMCOL put out a market breakdown in 1996 for diaper material costs, which can be seen below:
BREAKDOWN OF DIAPER MATERIAL COST
DIAPER MATERIAL % OF TOTAL MATERIAL
COSTS PER DIAPER
FLUFF PULP 19.7
SAP 27.7
BOTTOM SHEET 9.5
NONWOVEN TOP SHEET 15.5
CARRIER TISSUE 2.3
LYCRA MATERIAL OR RUBBER 2.1
MULTI-PURPOSE ADHESIVE 2.5
ELASTIC ADHESIVE 1.8
TAPE TAB PRESSURE SENSITIVE 5.5
OTHER 13.4
COURTESY OF NONWOVENS MARKETS, MAY 1996
(source: AMCOL website)
Design Criteria:
In the early 1980’s, the use of superabsorbent polymers in diapers grew into the mainstream. Diapers were first designed solely to optimize their absorptive ability. This required superabsorbents having a low crosslink density.1 However, this also resulted in small gel modulus when swollen, causing a "gel-block." These are swollen masses of gel that blocked the incoming liquid from entering the interior of the diaper. Gel-blocked masses were more likely to allow the urine to contact the baby’s skin for long periods of time, and were more likely to leak. To prevent this, new composite structures containing both cellulose pulp fluff and superabsorbent polymer were devloped. This providexd a matrix in which liquid could flow. In addition, the crosslink density of the gel particles was increased, resulting in products with a higher liquid retention under shear, despite a lower capacity. Although the equilibrium swelling capacity was somewhat lower, the improved gel modulus led to an overall improvement in diaper performance.1
Further design optimizations were introduced as the diaper industry became increasingly competitive. As the understanding of anatomy increased, the polymer was placed more in the front and back of the user, instead of in the crotch. In addition, ridged and lengthwise folds were developed to prevent leakage. Layers of different density were placed in the pad, allowing the liquid to move and store more efficiently. Layering has become more prevalent with the incorporation of superabsorbent polymer into absorbent cores. 1
Design Core:
Many aspects must be considered in the design of the polymer matrix in the diaper core. The absorption rate of the diaper must not be slower than the urination rate of the baby, otherwise leakage will occur. The absorption rate of the composite is influenced by the absorption rate of the superabsorbent polymer. On the other hand, fast swelling of the polymer may or may not be desirable. In some diaper designs, fast swelling may cause the diaper to leak if the porosity and permeability of the composite is reduced.1 The absorption rate of superabsorbent polymers is affected by the maximum absorption capacity of the polymer and its particle size and shape. The placement of fast or slow absorbing polymers in the composite structure therefore has important implications for the effectiveness of the composite. Many different schemes for mixing fluff and superabsorbent polymer have been investigated in order to find optimum diaper performance. In addition, particle size, placement, and relative amounts play a large role in the optimization of absorption. When the superabsorbent swelling is delayed in the wetting region of some diaper designs, there is more time to distribute urine through the diaper. By distributing the liiquid better throughout the diaper, there is less saturation of the core in the wetting region, so further wetness may be absorbed.
With a refined understanding of the impact of superabsorbent polymer on the absorbent core in the early 1990s ultra-thin diapers became possible. The amount of cellulose pulp fluff used in these diapers was reduced by half, yielding a thinner diaper with a higher concentration of superabsorbent polymer in the absorbent core.2 As polymer properties become increasingly understood, diapers become thinner as the ratio of polymer to fluff increases.
A separate layer of non-woven fibers was added to improve urine distribution in the diaper. This distribution layer was placed between the composite absorbent core, which consists of the cellulose fiber and superabsorbent, and the porous cover sheet. The distribution layer had lower absorbency than either the standard cellulose fluff or superabsorbent polymer and a lower density, which allowed for a fast liquid distribution within the diaper. The layer was made from either chemically crosslinked cellulose fibers or a nonabsorbent non-woven material, such as polypropylene fiber, and it was sufficiently porous to allow liquid to pass through freely.1
Construction of the SAP:
Superabsorbent polymer is added to baby diapers in basically two ways: layered or blended. Japanese diaper manufacturers commonly adopt the layered application. In this method, powdered superabsorbent polymer first is scattered onto a layer of fluff pulp. The fluff is then folded, so that the polymer is located in a centralized layer in the absorbent structure. This structure is covered with a non-woven fabric layer. In the blended application, the superabsorbent polymer first is mixed homogeneously with the fluff pulp. Then the mixture is laid down to give the absorbent structure, which is subsequently covered with a non-woven fabric. The blended application of the SAP is representative of American diaper manufacturers.
In each case, containment of the powdered polymer within the loose, porous structure of the diaper is a concern. A recent development in Japan is the use of thermally bondable fibers within the absorbent structure to help fix the superabsorbent in place. In this method, some of the fluff pulp is replaced with thermally bondable fibers.1
CHEMICAL PROPERTIES
Structure – Property Relationship:
The structure of Polyacrylic acid is as follows, and contains an ionizable group on each repeat unit (-COOH).6
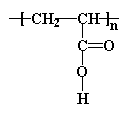
These polymer chains are then crosslinked at the –OH.
The mechanism of swelling of ionized, crosslinked polymer networks is based upon the concept of osmotic pressure. According to Flory9, the polymer acts as a semipermeable membrane which does not allow charge substituents to exit the polymer into the surrounding solution. This is because the ionized monomeric units contain fixed charges which attract and fix ions from the outer solution. Therefore, a charge gradient is set up, in which the concentration of free ions is greater outside of the polymer. Therefore, the osmotic pressure exerted by the gradient causes the polymer chain to swell as further ions diffuse in.
Polymer Synthesis:
The synthetic pathway of polyacrylic acid is shown below:
Na2S2O8 is a radical initiator, which polymerizes the sodium acrylate salt monomers. The crosslinking agent is added in the same step.1
Swelling Capacity:
Certainly the first property and arguably the most important in a commercial superabsorbent used in the personal care market is the extent of swelling. This is true not only because swelling is related to the properties of the network, but also because the principal performance criterion for diapers is the amount of liquid contained per unit cost of diaper.1 In which case, the swelling capacity is approximately 20-40 mL of urine per gram of polymer.2
If the superabsorbent polymer is more highly crosslinked, it is more rigid in the swollen state. Improving the rigidity of the particles enables the swelling particles actually to push aside the fiber component of the composite, thereby maintaining the porosity and permeability during subsequent contacts with liquid. However, this must be optimized, as particles which are too rigid will cause leaks by tearing the surrounding fiber.
How Absorption Works:
Superabsorbent polymers are crosslinked networks of flexible polymer chains. The most efficient water absorbers are polymer networks that carry dissociated ionic functional groups. Except for the molecular-sized chains that make up the network, this picture of a network is remarkably similar looking to the mass of cotton fibers. The difference is that cotton takes up water by convection – water is "sucked" up, wetting the dry fibers; SAPs work by diffusion on the molecular level, since their "fibers" are actually long chained molecules.
Water diffuses into a particle of superabsorbent polymer when the concentration of water is initially lower in the interior of the particle. As water travels into the particle, it swells to accommodate the additional molecules. Because the polymer molecules are crosslinked, they do not dissolve in the absorbing liquid.
Absorbency under load and stability of the gel against shear are important properties of superabsorbent polymers and relate strongly to diaper performance. Diaper leakage was closely correlated to the stability of gel to shearing. More rigid superabsorbent particles, created by increasing the crosslinking, allows for a higher gel modulus and helps the particle withstand the shearing from the baby’s weight.
Granules:
The most commonly available superabsorbent polymers are hard, dry, granular powders that look much like clean white sand or granular table sugar. When these polymer particles are placed in water, a slurry of water and the particles is formed. Gradually the superabsorbent polymer absorbs the water, turning into a soft, rubbery gel. On average, fluffed cellulose pulp fibers will absorb about 12 g of water per gram of dry fiber, whereas superabsorbent polymers will absorb up to 1,000 g of water per gram of polymer. 1
Crosslinkers:
Small amounts of crosslinkers play a major role in modifying the properties of superabsorbent polymers. In addition to modifying the swelling and mechanical properties, the crosslinker affects the amount of soluble polymer found during the polymerization as result of its relative reactivity with acrylic acid or sodium acrylate. Efficiency of crosslinking will also depend on steric hindrance and reduced mobility at the site of pendant double bonds, the tendency of a given crosslinker to undergo intermolecular addition reactions, and the solubility of the crosslinker in the monomer mixture.1
Surface Tension:
In a diaper core, capillaries exist between fluff pulp fibers, polymer particles, or the two in combination. Distribution of liquid in the diaper core will be affected by the surface tension of the liquid that is flowing in these capillaries. In use, impurities may be extracted from a swollen superabsorbent polymer into the solution external to it. Through the surface tension of the solution, the movement of fluid through the capillaries in a diaper core may be affected by these impurities.2
Temperature Change:
Superabsorbent polymers are used under conditions in which the system temperature may change over time. For example, in a diaper, the superabsorbent polymer will first be bathed in a salt and urea solution that is at the internal temperature of the human body, but the resulting gel will cool slowly in contact with the external environment. The extent and rate of cooling will depend on the climate and other environmental factors. The diffusion coefficient of polymers in solution is temperature dependent, and this should be reflected in the absorption rate of superabsorbent polymers.1
ENVIRONMENT
Overall:
On account of 90% of all superabsorbent materials are used in disposable articles, most of which are disposed of in landfills in the United States or by incineration in northern Europe, there is a perceived environmental problem with superabsorbent polymers. In the late 1980s bans or taxes on disposable diapers were being considered in at least 20 states. However, analysis of both disposable and cloth diapers manufactured in the late 1980s have shown that there is no clearly superior choice in terms of environmental impact.
Since then, disposable diapers have been modified to use fewer raw materials, which should result in a reduced solid waste burden, reduced packaging costs, and reduced transportation costs. Despite the technical analysis, consumers clearly perceive disposable absorbent products, specifically diapers, as having a negative impact on the environment. Therefore, superabsorbent polymer producers have been interested in developing biodegradable diaper or other absorbent product. Articles incorporating biodegradable superabsorbents might be disposed of in municipal composting facilities or flushed down the toilet to degrade in domestic septic tanks or at municipal wastewater treatment plants. Several diapers claiming biodegradability have been marketed, but none has enjoyed commercial success.
Furthermore, the advantages of a biodegradable superabsorbent polymer will be realized fully only in conjunction with a completely biodegradable structure, for example, diaper with biodegradable back sheet, tapes, adhesives, and elastics.1
Amount of Landfill:
In one infant’s lifetime, approximately 8,000-10,000 disposable diapers will be used where each one of those diapers takes approximately 500 years to degrade in a landfill. 7 These diapers are filled with untreated body excrement. Over five million tons of it, that could be carrying intestinal viruses, is brought to landfills. Groundwater contamination could be attributed to this form of disposal since insects are attracted to the sewage where they may be carrying diseases that can be transmitted. In 1990 alone, over 18 billion disposable diapers were thrown into the US’s landfills which where not readily biodegradable. The diapers themselves need to be exposed to air and sun to allow the paper to decompose. It also must be taken into consideration that thirty percent of a disposable diaper is plastic and is not compostable. Recycle plants can only handle 400 of the 10,000 tons of diapers, which are not completely recyclable, in landfills each day.8 This number would only hold if they didn’t have to process any other compostable garbage.
CONCLUSION:
There are several reasons for the continued development of advanced superabsorbent polymers. Diapers manufacturers would like to reduce their manufacturing costs. A superabsorbent that can take on other roles in the absorbent core can displace other components in the core, in turn, reducing raw material costs and simplifying construction. For example, a superabsorbent fiber that can provide rapid fluid absorption and adequate wicking and transport of fluid could displace cellulose fluff in the absorbent core, leading potentially to a simpler or less expensive diaper. On the other hand, manufacturers of premium diapers seek to differentiate their products in order to command a higher price. An advanced superabsorbent that presents marketable performance advantages, for example, biodegradability to an absorbent product may be of special value to diaper manufacturers.
References:
www.ecomall.com/greenshopping/diaper2z.htm
9. Flory, Principles of Polymer Chemisty, Cornell U. Press, 1953.